Study under review: Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods.
In Study Deep Dives #6, available to Examine Members, Margaret Leitch discussed the neurobiology of eating behavior. Basically, the food industry is great at bringing out the best in food, making it “hyper-palatable” to consumers. Chronic exposure to hyper-palatable food can lead to changes in brain chemistry, similar to changes observed with drug use. People literally become addicted to these foods and can experience withdrawal symptoms when they stop eating them.
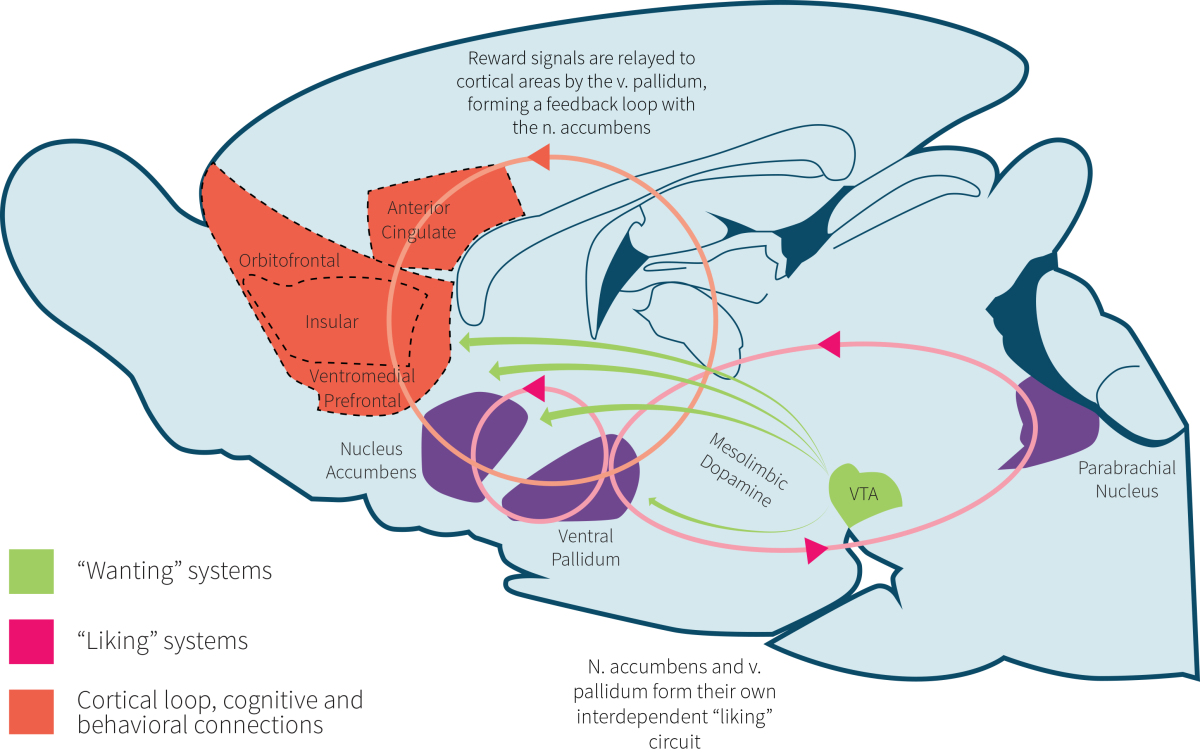
Our liking of food and the reward system involved with hyper-palatability is believed to involve a combination of brain regions (shown in Figure 1) involved in the regulation of emotion, decision making, and impulse control: the hypothalamus, nucleus accumbens, amygdala, anterior insula, caudate nucleus, and orbitofrontal cortex (OFC). These regions react in response to numerous gut hormones produced in the stomach, small intestine, pancreas, and colon, as well as visual and olfactory (smell) cues.
For instance, glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) are secreted by cells within the ileum (end portion of the small intestine) and within the colon in response to food, especially carbohydrates and fats. Infusion of GLP-1 reduces energy intake in both lean and overweight individuals, an effect believed to be caused at least partly by GLP-1 affecting the hypothalamus and caudate brain regions. Infusion of PYY has also been shown to reduce food intake and hunger in lean and obese people.
While there has been a tremendous amount of research investigating how these and other gut hormones impact the brain’s regulation of appetite, emerging research suggests that not all potential brain modulators are made by us. One massive part of the digestive system that may influence satiety is the gut microbiome. Evidence in rodents suggests that resistant starch (a type of fermentable fiber) supplementation influences appetite through effects on gene expression and the brain. Similar findings have also been observed with the fibers beta-glucan and inulin.
What the above fibers all have in common is that they are metabolized by the gut microbiome, producing short-chain fatty acids (SCFAs) as a byproduct. The primary SCFAs are acetate, propionate, and butyrate. Preliminary animal research suggests that circulating acetate crosses the blood-brain barrier to directly influence appetite regulation centers. Additionally, the SCFAs have been shown to promote the release of several gut hormones that promote satiety through their influence on the brain.
Recently, human research has shown that delivering propionate directly to the colon increases the secretion of PYY and GLP-1 and reduces energy intake at a buffet meal. It also significantly reduced weight gain over a 24-week supplementation period. This was done with an inulin-propionate ester (IPE), whereby propionate is bonded by an ester linkage to inulin, a carrier molecule. The ester linkage is broken down by the gut bacteria, which results in the delivery of propionate directly to the colon.
The study under review was a follow-up to the above IPE research. It sought to examine the effect of an acute increase in colonic propionate production on energy intake and brain regions involved with reward processing and eating.
Satiety and food reward are an intricate dance between numerous gut hormones, the gut microbiome, and the brain. Previous research has shown that increasing the amount of the short-chain fatty acid propionate in the human colon reduces weight and appetite. The current study sought to examine how increasing propionate influences energy intake and brain activity.
Who and what was studied?
Twenty healthy men participated in this randomized, placebo-controlled, single-blind crossover study. The majority were middle-aged white Europeans (90%) with an average BMI of 25 (overweight) and an average body fat percentage of 20.6%.
The participants attended two separate study visits at least six days apart. Upon arriving, baseline measurements of weight and body composition were collected with a bioelectrical impedance device. Additionally, blood samples for the analysis of glucose, insulin, GLP-1, PYY, and SCFAs, breath hydrogen samples for the analysis of fermentation by the microbiome in the colon, and visual analog scale ratings for mood and appetite were collected every 30 minutes throughout the study period.
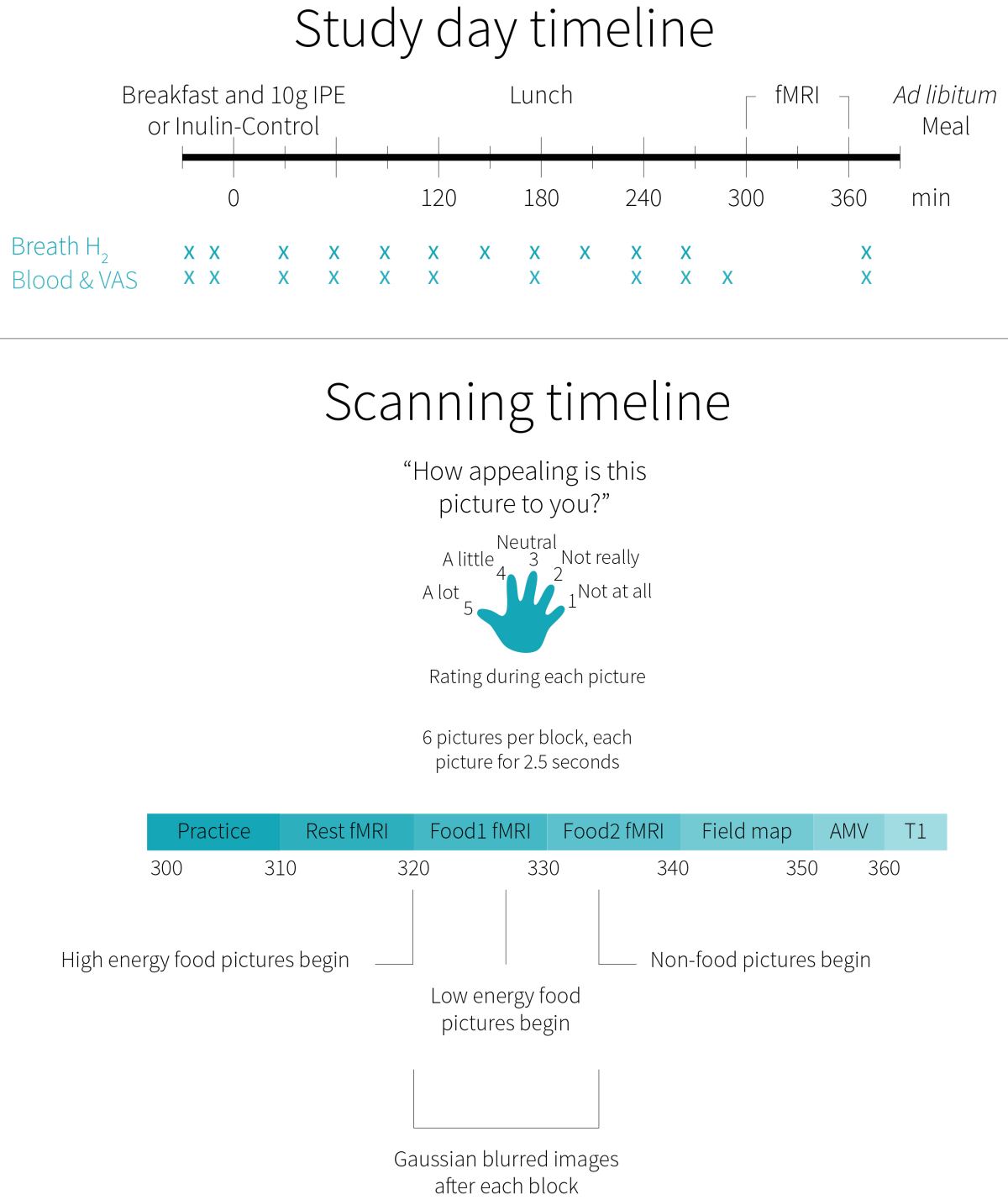
The study timeline is summarized in Figure 2. At time-point zero, a standardized chocolate milkshake and snack bar breakfast containing either 10 grams of IPE or 10 grams of inulin (control) was provided to the participants. At 180 minutes, the participants consumed a cheese sandwich and snack bar lunch. At 300 minutes, the participants underwent a 60-minute fMRI session, based on the previous IPE research showing that successful delivery of IPE to the colon occurred after 240 minutes. Finally, a tomato and mozzarella pasta casserole was provided to the participants at 390 minutes (30 minutes after completing the fMRI), who were instructed to eat until they felt comfortably full.
The fMRI session included, in order, a practice run with animals, a resting-state analysis, a food picture task, and an auditory-motor-visual (AMV) task. The food task provided the brain imaging data used in this study. It presented photographs of high-energy and low-energy foods, as well as non-food-related household objects and blurred images of all the aforementioned photos. While each image was on display, the participants were asked to rate how appealing each picture was to them on a Likert scale of one to five. It was determined, a priori, that participants with a response rate less than 90% would be excluded from analysis. The AMV task was a control task to exclude potential changes in brain activity between visits.
Twenty healthy males attended two study visits, where they consumed 10 grams of IPE or inulin (control) alongside a standardized breakfast meal. They then consumed a standardized lunch and underwent fMRI scans to measure brain activity. Finally, they were fed a pasta meal to assess unrestricted food intake. Blood samples and appetite ratings were collected throughout.
What were the findings?
In both groups, breath hydrogen concentrations were significantly elevated above baseline from 210 minutes to the end of the study, indicating that both the IPE and inulin control supplements reached the colon after this time and were successfully fermented by the microbiome. However, the inulin control group had levels significantly greater than the IPE group, which may be owed to the greater amount of inulin supplied (10 grams vs. 7.3 grams in the IPE group).
There were no significant differences between groups for the AMV control task. When undergoing the food task, two of the 20 participants were excluded because they failed to rate at least 90% of the images. For the remaining participants, several differences in brain activity were observed, but statistical significance was only achieved for the caudate and nucleus accumbens. Additionally, the IPE group had significantly lower caudate activity when viewing high-energy foods but not low-energy foods.
Supporting the above findings, ratings of high-energy foods were significantly lower in the IPE group, compared to the control group. Additionally, the IPE group had a significantly increased reaction time to the food pictures.
During the final meal, five of the participants had their data removed from analysis because they consumed all the food presented during at least one visit. For the remaining 15 participants, the IPE group consumed significantly fewer calories than the control group (711 vs. 810 kcal). There were no significant differences between groups for changes in blood glucose, insulin, GLP-1, PYY, or SCFAs throughout the study period. For the individual SCFAs, both groups experienced a significant increase in serum butyrate concentrations at 240 minutes, but not in acetate or propionate concentrations.
There were no significant correlations between the amount of calories consumed, appetite, or high-energy food picture ratings and brain activity in the caudate, nucleus accumbens, or both combined. However, greater appetite ratings were correlated with greater caloric intake during the final meal.
The IPE group, as compared to the control group, had significantly reduced brain activity in the caudate and marginally reduced brain activity in the nucleus accumbens in response to images of high-energy foods. Additionally, the IPE group rated high-energy foods as significantly less appealing and consumed significantly fewer calories. However, no significant differences between groups were observed for changes in blood glucose, insulin, GLP-1, PYY, or SCFAs.
What does the study really tell us?
The current study sought to evaluate how elevated levels of colonic propionate influenced brain activity among several brain regions previously associated with food-reward processing and eating behavior. The results showed that some, but not all, of the regions investigated were less responsive to high-energy food pictures. Additionally, the participants perceived high-energy foods as less appealing and ate fewer calories during an unrestricted pasta meal.
The researchers were confident that delivery of the IPE and control to the colon occurred before the fMRI imaging tasks because breath hydrogen levels were significantly increased at that time, which is indicative of bacterial fermentation in the colon. The authors note that the 10-gram dose of IPE used in this study delivers 2.36 grams of propionate, which is 2.5 times typical daily propionate production of the typical low-fiber Western diet. However, while the current study suggests that increased colonic propionate levels may modulate the brain-reward circuitry, it doesn’t tell us how.
No differences between groups were noted for changes in total blood SCFAs or butyrate, acetate, and propionate specifically. In fact, propionate didn’t change significantly from baseline in either group. However, this may be due to a lack of statistical power to detect a significant change, as the sample size was small (18 men) and no a priori analysis was conducted to determine how many people would be necessary to see a significant change. Additionally, about 90% of absorbed propionate is extracted by the liver, meaning very little actually makes it into circulation. Nonetheless, circulating propionate concentrations nonsignificantly increased by 13-15% in both groups, suggesting that a direct action on the brain cannot explain the difference in brain activity between groups. At least not as far as we can tell, as the low sample size may not allow us to see any differences that may exist.
Concentrations of blood glucose and insulin were similar between groups, which suggests propionate did not influence insulin sensitivity or the metabolic response to the meals in a manner that could, in turn, impact brain satiety signals. Additionally, two important satiety gut hormones, GLP-1 and PYY, were not different between groups. There are other important gut hormones associated with reducing food intake that were not measured in the current study, such as CCK, oxyntomodulin, and amylin. It is possible that increased colonic propionate may have influenced brain activity indirectly, through increasing the secretion of these hormones.
The current study had a strong randomized crossover design that provides a high degree of reliability on the integrity of the findings. However, the small sample of primarily Caucasian overweight men warrants caution with generalization and the single-blind nature of the design introduces the potential for bias. Further research involving other ethnicities, women, and people who have obesity is needed.
The use of fMRI imaging is both a strength and a limitation. Certainly the procedure used is a well-validated and noninvasive measure of brain activity. However, fMRI imaging relies on software that uses statistics to generate the high-resolution brain images and determine changes in brain activity. Recently, a study has suggested that the most common software packages for fMRI analysis (including the one used in the current study) can result in false-positive rates of up to 70%. Thus, although the design itself may have integrity, the findings may not necessarily be accurate.
Still, the finding that high-energy foods were significantly less appealing and food intake was significantly lower do show that, regardless of brain region activity, the IPE did impact perceptions of food and subsequent intake in a beneficial manner. Further research investigating any possible dose-response relationship and involving a wider range of populations is warranted.
Increased concentrations of propionate in the colon are associated with modified brain activity, reduced appeal of high-energy foods, and reduced food intake. However, these associations do not appear to be explained by changes in circulating SCFA or gut hormone concentrations. Further research is needed to investigate how increased colonic propionate leads to these observed outcomes.
The big picture
The modern food environment is rich in relatively inexpensive, highly palatable, energy-dense foods, as well as cues, such as advertising, that promote food intake. Although the role of this environment in weight gain and obesity is not fully understood, enough evidence has accumulated to prompt some researchers to urge changes in public policy that target food companies. As the title of one review eloquently puts it: the tempted brain eats, and overconsumption of calories is thought to be driven, at least in part, by a more hedonic form of eating.
As touched on in the introduction, several regions of the brain are involved in hedonic eating and food-reward. The past two decades have produced important evidence about how perturbations in these brain regions may occur in obesity (1, 2, 3) or lead to weight gain (1, 2, 3). Accordingly, research like the current study is an important next step for the prevention and treatment of obesity. That is, if researchers know that the brain’s food-reward circuitry is likely involved in overeating, what is needed to restore proper functioning of that circuitry? Could this help reduce hedonic overeating?
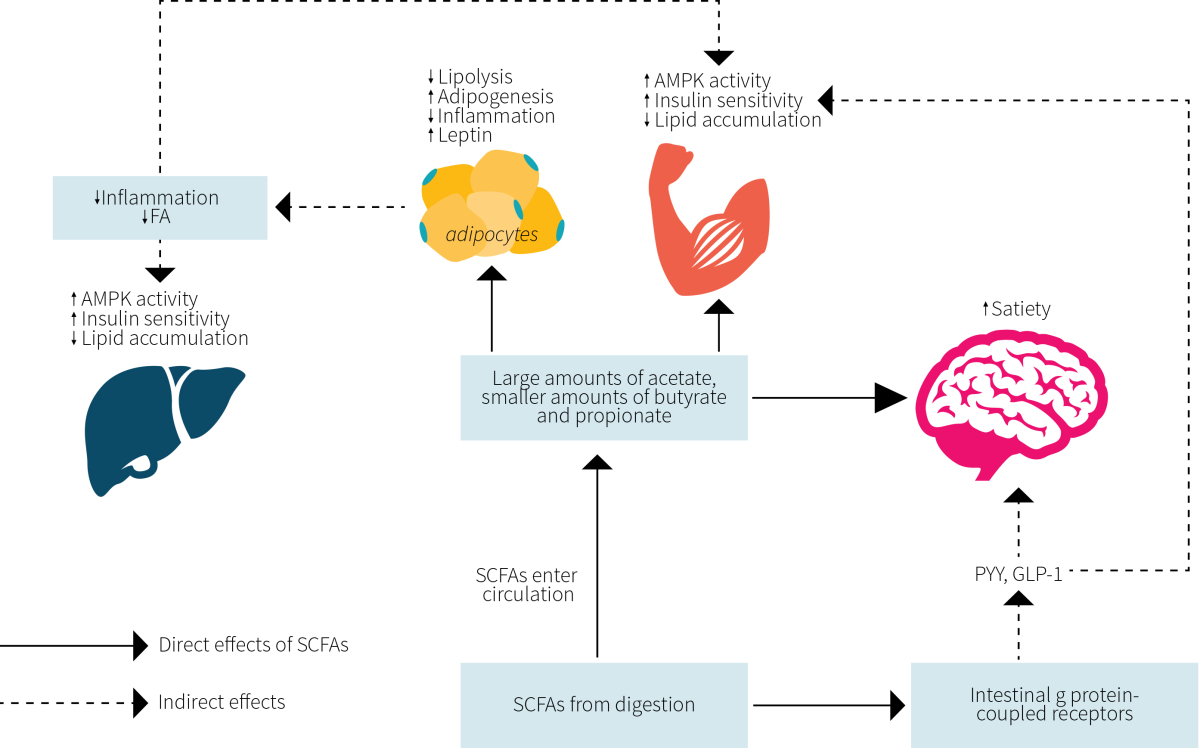
Turning to SCFAs for an answer is not far-fetched. Accumulating evidence suggests that SCFAs play a prominent role in regulating metabolism, inflammation, and disease. As shown in Figure 3, experimental evidence from humans, animals, and cell cultures show that SCFAs are able to directly influence fat tissue, muscle tissue, the liver, and the brain where they coordinate to reduce fat accumulation and inflammation while promoting insulin sensitivity and satiety.
The current study is the first to show that one of the SCFAs, propionate, acts to modulate the brain’s food-reward circuitry in a way that may explain its ability to reduce caloric intake. When considered alongside the current evidence-based for SCFAs, these tiny molecules are appearing to be an ally in the battleground of the modern food environment.
The current food environment is designed to promote overeating. In addition to their known health-promoting effects, SCFAs have now been implicated in reducing energy intake.
Frequently Asked Questions
Q. Would a high-fiber diet have a similar effect as the IPE treatment?
It is possible, although a future study will be needed to answer this question with confidence. However, most American adults do not meet recommended fiber intakes as is, raising questions to the sustainability of such an intervention. The authors of the study under review mention that the current 10-gram dose of IPE leads to 2.5 times the amount of propionate normally produced. If the average American is consuming 16 grams of fiber per day, would it be reasonable to have them consume 40 grams per day? What about the uncomfortable side effects of gas and bloating? These questions have not been resolved by research as of yet.
What should I know?
Increased concentrations of the short-chain fatty acid called propionate in the colon is associated with reduced brain activity in response to the viewing of high-energy foods, a reduction in the subjective appeal of high-energy foods, and a reduced intake of calories. This study is the first to show that SCFAs modulate brain circuitry involved in food-reward processing and adds to the evidence-base that these molecules may be a super soldier in the fight against weight gain and disease.
