The optimal diet for treating metabolic diseases like obesity and type 2 diabetes remains controversial, with evidence suggesting a variety of nutritional strategies that can be effective so long as people adhere to them. However, some interventions may be more effective than others. One strategy that’s gaining steam involves eating more protein.
Even though the term “high-protein diet” varies in definition from study to study, evidence to date supports the idea that eating more protein than the recommended daily allowance of 0.8 grams per kilogram bodyweight results in less hunger and a reduced appetite, increased energy expenditure, and a preservation or increase of lean body mass. A recent meta-analysis of 74 randomized controlled trials showed that eating a higher protein diet (27% vs. 18% of calories on average) significantly reduced several cardiometabolic risk factors, including body weight, BMI, waist circumference, blood pressure, triglycerides, and fasting insulin, while also significantly increasing HDL-cholesterol and satiety. A strong body of evidence supports the claim that a high-protein diet can facilitate dietary adherence, health improvement, and long-term fat loss.
But it’s possible that not all protein is the same when it comes to health. A meta-analysis of observational research totaling over half a million people suggests that animal protein is associated with an increased risk of developing type 2 diabetes, while plant protein is not. However, plants are bundles of fiber and bioactive compounds that could explain the risk difference, and people who eat more veggies typically have a healthier lifestyle. These confounders make it impossible to relate differences in type 2 diabetes risk to differences in plant and animal protein, per se.
In general, animal proteins are higher in branched-chain amino acid content (BCAAs; leucine, isoleucine, and valine) and sulfurous amino acids (methionine and cysteine) than plant proteins. The role that these amino acids play in the pathology of type 2 diabetes is contested. Dietary methionine restriction has been shown in animals to increase insulin sensitivity. Similarly, BCAAs, especially leucine, are potent stimulators of the mTOR pathway, and chronic stimulation of mTOR has been implicated in insulin resistance. Some evidence suggests that the metabolism of BCAAs is disrupted in people with type 2 diabetes, resulting in an exacerbation of insulin resistance (as shown in Figure 1), and dietary intake of BCAAs has been associated with the risk of developing type 2 diabetes.
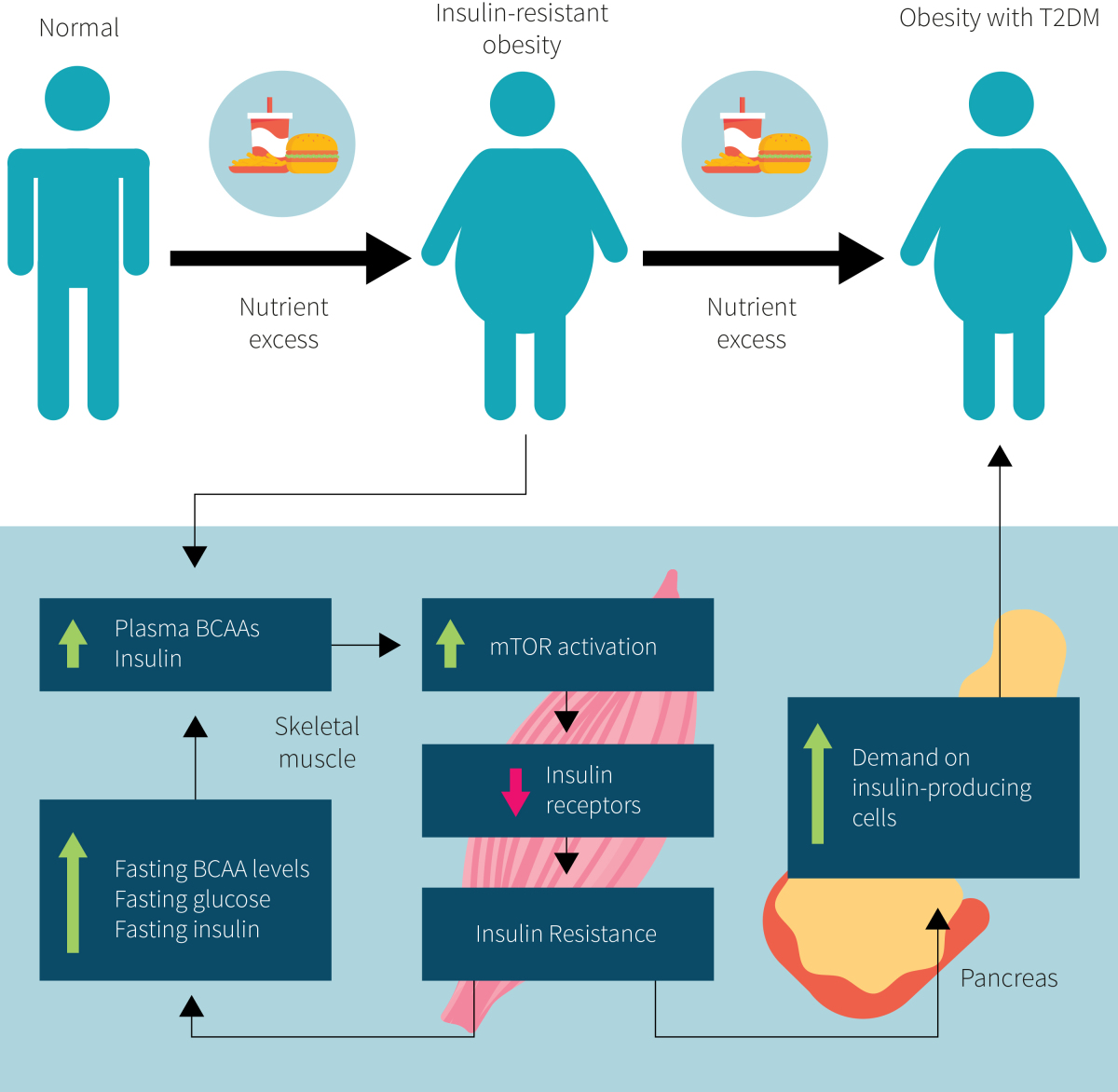
On the other hand, animal studies suggest that mTOR activation in the brain reduces food intake and bodyweight. These effects and brain mTOR signaling are brought about through increased dietary leucine intake. Moreover, BCAA consumption, especially isoleucine, has been shown to have beneficial effects on glucose metabolism.
To investigate how the amino acid composition of a diet influences health outcomes in people with type 2 diabetes, the study under review examined the effects of a high-protein diet containing predominantly either animal or plant protein on insulin sensitivity in participants with type 2 diabetes.
High-protein diets are well established to promote fat loss and health improvement in people with obesity and type 2 diabetes. How differences in the amino acid composition of plant and animal proteins may affect health remain less well investigated. The study under review compared the effects of a high-protein diet containing mostly animal protein or plant protein in people with type 2 diabetes.
Who and what was studied?
This was a randomized controlled trial in which adults with diagnosed type 2 diabetes and an HbA1c between 6-11% were recruited to consume either a high-protein diet based on animal protein or a high-protein diet based on plant protein for six weeks. Forty-four people began the intervention and were randomized by matching for age, sex, BMI, HbA1c, and diabetic drug use, but only 37 people completed the intervention, with a similar dropout rate between groups. The participants had an average age of about 64 years, an average HbA1c of about 7%, and an average BMI of about 30. Only eight were taking no medications, while the remainder were using metformin alone or in combination with another drug (most common being a DPP-4 inhibitor).
All participants had their total daily energy expenditure estimated by measuring basal metabolic rate via indirect calorimetry. Researchers also accounted for self-reported physical activity levels. Calculated energy expenditure was compared to the average daily energy intake obtained through a five-day food log that each participant completed before beginning the intervention. Combined, these values were used to create individualized diet plans for each participant that attempted to maintain their bodyweight.
All participants received a diet plan that provided 30% of the calories as protein (about two grams per kilogram of bodyweight), 30% as fat, and 40% as carbohydrate. However, the animal protein group obtained their protein primarily from dairy products and meat, while the plant protein group obtained their protein primarily from pea protein that was incorporated into specific foods (e.g. mashed potatoes, bread, and noodles). Ultimately, the animal protein group consumed 80% of their protein from animal products and the plant protein group consumed 72% of their protein from plants.
Participants received about half of their food from the food plans every two weeks to facilitate compliance, and were provided a detailed substitution list to allow for more flexibility and variation. Additionally, each participant was weighed when they picked up their food and the food plans were adjusted to maintain bodyweight, if necessary. The participants were asked to weigh and record all food eaten, including any deviations from the food plans.
At baseline and again after the six-week intervention, the participants underwent a hyperinsulinemic-euglycemic clamp (as outlined in Figure 2) to determine insulin sensitivity, which was the primary outcome. Several secondary outcomes were also assessed, including blood pressure, blood lipids, serum markers of glycemic control, C-reactive protein, and blood and urinary markers of kidney function. Data regarding body composition, liver fat, blood lipid and amino acid composition, inflammatory markers, and gene expression were published previously.
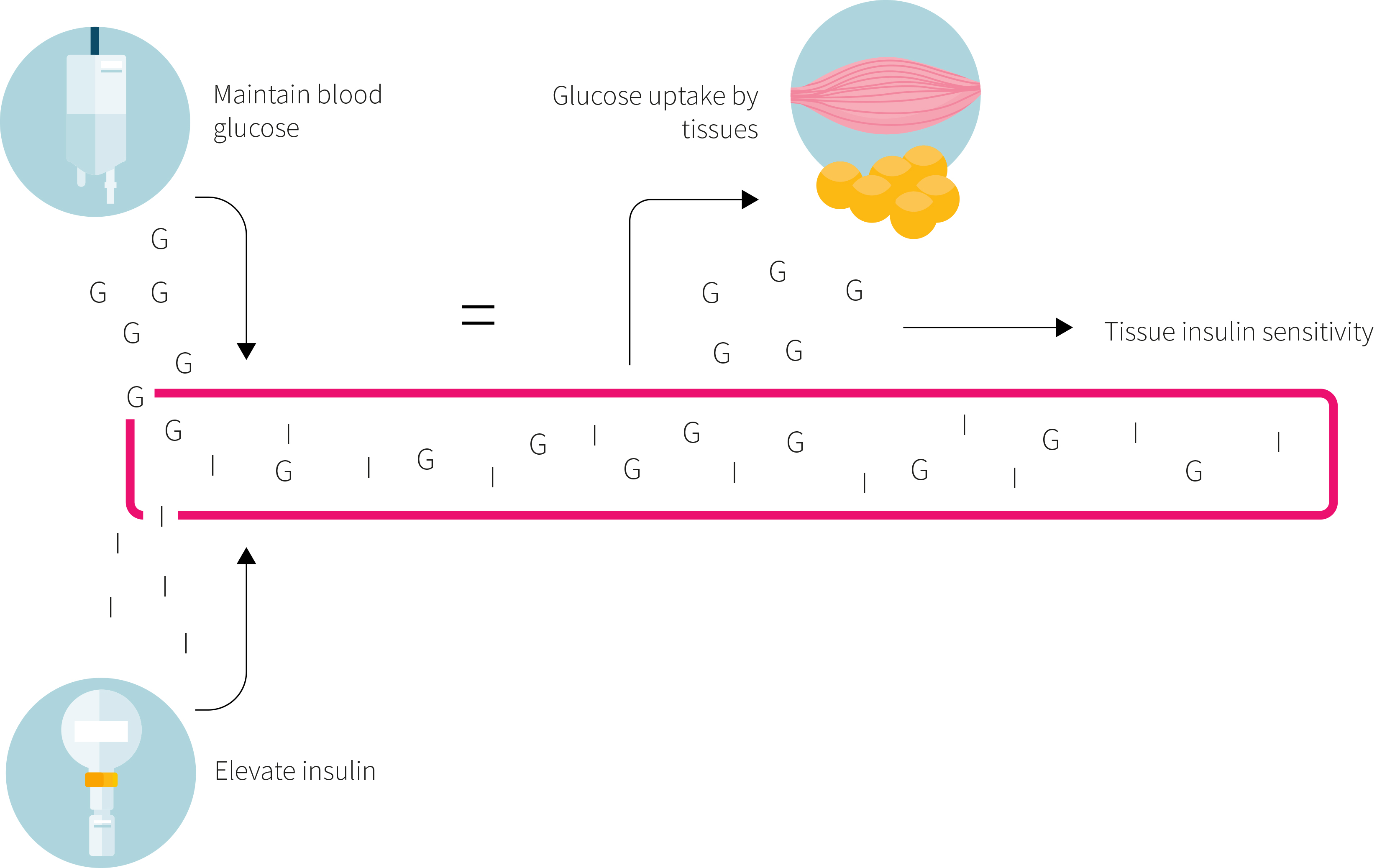
This was a randomized controlled trial in which 37 people with type 2 diabetes consumed a weight maintenance diet providing 30% of the calories as protein, 30% as fat, and 40% as carbohydrate for six weeks. One group consumed most of their protein from dairy products and meat while another group consumed most of their protein from pea protein. The primary outcome was the difference between groups in the change in insulin sensitivity.
What were the findings?
There were no significant differences between groups for insulin sensitivity, any other cardiometabolic risk factor, or markers of kidney function after the intervention. Based on the previously published data, there were also no significant differences between groups for changes in body composition, liver fat, blood lipid and amino acid composition, inflammatory markers, or gene expression.
Dietary adherence to the food plans was strong and food logs suggested that the actual ratio of macronutrients was within 1% of the planned ratio. However, despite attempts to maintain bodyweight, both groups showed a similar and significant, albeit small (0.5-0.8 BMI) reduction during the intervention. Changes in body composition did not correlate with insulin sensitivity or any other outcome.
There was no significant difference between groups for insulin sensitivity, blood pressure, blood lipids, serum markers of glycemic control, C-reactive protein, and blood and urinary markers of kidney function.
What does the study really tell us?
The study under review tells us that the amino acid composition of the diet does not affect insulin sensitivity or markers of cardiometabolic health and kidney function. Importantly, by incorporating pea protein into consumed foods instead of increasing the consumption of plants, this study avoided confounding from the fiber and bioactive compounds that may influence health outcomes. Instead, it directly tested the effect of the diet’s amino acid profile.
Importantly, the study methodology limits its external validity, since most people obtain their plant protein through eating plants rather than fortifying their food with pea protein. But this isn’t necessarily a bad thing. The authors did not necessarily care about external validity and were trying to answer a more basic, specific question: does amino acid composition matter for insulin sensitivity? Now, in future research, if we notice differences in insulin sensitivity between plant-based and animal-based diets, we can more confidently say that the amino acid composition probably played little role.
The findings of this study support those of a study discussed in NERD #12, Volume 1, Throwdown: plant vs animal protein for metabolic syndrome. In this earlier study, people with metabolic syndrome were randomized to follow a modified DASH diet rich in either plant protein or animal protein for a five-week weight maintenance phase, a six-week weight loss phase, and a 12-week free-living phase. Over the entire six-month intervention, there were no differences in any outcome between the animal and plant protein groups. Unlike the current study, however, the plant protein group ate actual plants to obtain their protein and the overall protein intake was lower, at around 18% of calorie intake.
Both interventions in the study at hand contained about 10% more kcal from protein and 10% less kcal from fat than the habitual diets of the participants (carbohydrate intake was similar). Both groups reported significant weight loss, which could be due to protein’s established beneficial effects on satiety. This weight loss may have influenced at least a couple outcomes, since there was a significant interaction between it and systolic blood pressure, total cholesterol, and LDL-cholesterol, all of which showed improvement over time within each group. Other markers that saw improvement over time in both groups were insulin sensitivity, HbA1c, fasting glucose, HOMA-IR, and C-reactive protein. However, there was no control group that maintained their dietary habits for comparison, the groups showed variable within-group significance, and no adjustment for multiple comparisons was performed, which greatly limits our ability to draw conclusions from findings outside of the fact that the groups did not differ from one another.
Notably, the previously published data from this study reported that neither diet significantly altered fasting plasma amino acid levels, but that the consumption of a test meal based on each group’s dietary parameters did. Specifically, the animal protein meal led to significantly greater increases in plasma BCAA and sulfurous amino acid concentrations than the plant protein meal. Even so, both meals resulted in similar increases in mTOR activation. This may be owed to the fact that both meals contained more than 30 grams of protein and three grams of leucine, which is above the threshold intake level suggested to maximally stimulate mTOR.
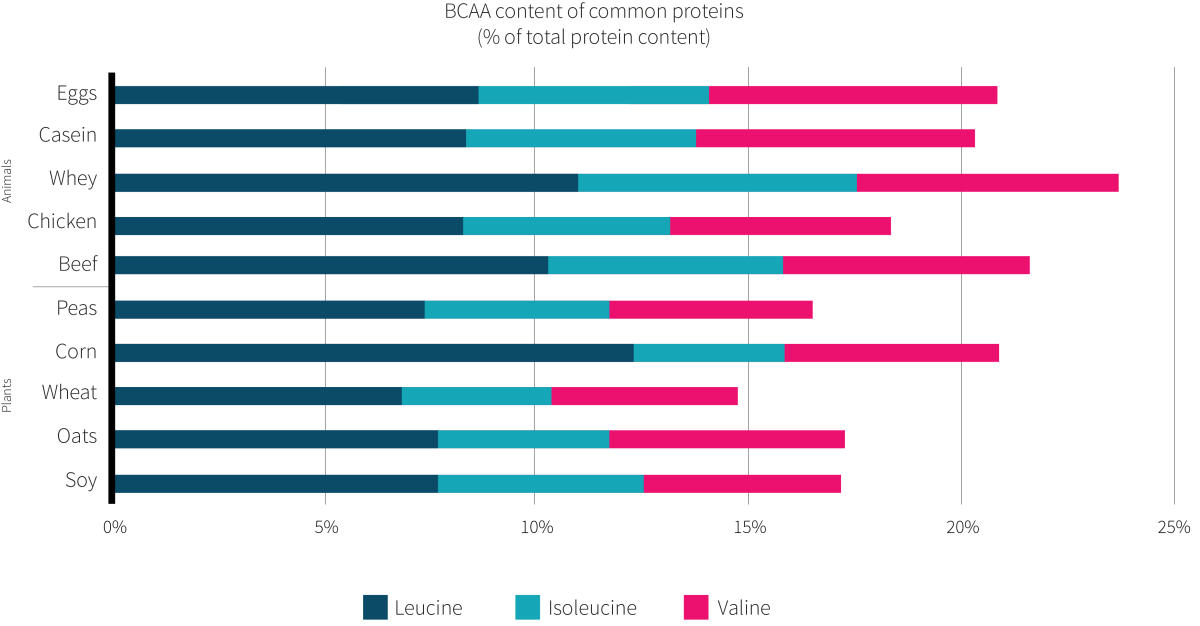
The choice of pea protein in the study at hand was odd, considering that it contains a similar amount of BCAAs as animal-based sources of protein (Figure 3 shows specific numbers). After all, one of the proposed mechanisms for why substituting plant proteins for animal proteins would benefit insulin sensitivity for people with type 2 diabetes is because of the lower BCAA content. Yet, pea protein is about 17% BCAAs, which is close to the 18-22% BCAA content of meat and eggs. Accordingly, the test diets were estimated to differ in BCAA content by only about six grams per day. Whether this is large enough of a difference to lead to differences in insulin sensitivity remains unknown, but the similar findings from the study discussed in NERD #12, Volume 1, Throwdown: plant vs animal protein for metabolic syndrome, which used whole-food plants and presumably had a larger difference in BCAA content between diets, supports the notion that the amino acid composition of the diet plays a small role.
Neither diet differed in their effects on markers of kidney function, which were largely benign. This is an important finding because diabetes increases the risk of developing kidney disease and protein restriction is the recommended medical nutrition therapy for managing kidney disease. Although this study was not designed to directly test the effect of protein intake on kidney health, its findings do support the body of evidence showing that eating a high protein diet is not detrimental to kidney function in people with healthy kidneys.
Other limitations of this study include the relatively short duration and small sample of older (mid-sixties) participants with diabetes. As already mentioned, the intervention itself also limits external validity, since most people are not getting two-thirds of their protein from peas. Still, a strength of the study is that its primary outcome, the effect of the different protein sources on the participants' insulin sensitivity, was measured using the gold-standard hyperinsulinemic-euglycemic clamp. This greatly increases confidence in the conclusion that plant and animal proteins do not differentially affect insulin sensitivity. However, there may still be other factors specific to plants that provide a health benefit when plant foods replace food sources of animal protein.
Using the gold-standard for determining insulin sensitivity, the study under review suggests that animal and plant proteins have similar effects on insulin sensitivity, as well as most other cardiometabolic risk factors. The real-world relevance, on the other hand, is questionable because the use of pea protein-enriched functional foods. Most people obtain plant protein through plant foods, and it is possible that other factors inherent to plant foods provide a health benefit when plant foods replace food sources of animal protein.
The big picture
Increasing insulin sensitivity via dietary means is a thoroughly investigated topic. Any diet that promotes fat loss is going to result in long-term benefits, and eating more protein is a viable way to increase dietary adherence and promote favorable changes in glucose metabolism. The study under review further suggests that the type of protein is largely irrelevant.
What cannot be ignored, however, is the way in which food sources of protein are prepared. NERD Issue #24, Volume 1, The high cost of high heat cooking, discussed a randomized controlled trial in which people with metabolic syndrome (but not type 2 diabetes) were randomly assigned to continue with their usual diet or to use gentler cooking methods in food preparation (boil, poach, stew, or steam rather than fry, bake, or grill) for one year. The premise was that harsher cooking methods increase the formation of advanced glycation endproducts (AGEs) that exacerbate insulin resistance. The study showed that consuming an AGE-restricted diet for one year led to significant reductions in insulin resistance and numerous markers of inflammation and oxidative stress. Although it remains unknown how these benefits translate into actual reductions in the risk of developing type 2 diabetes, this study does show that something as simple and usually overlooked as cooking method can have a significant effect on health.
The order in which we eat our meals can also influence our insulin sensitivity, even though most people don’t consider this in their daily dietary routines. NERD Issue #10, Volume 1, Carbs-protein or protein-carbs … does food order matter, addresses this topic by discussing the results of a small study involving people with type 2 diabetes who were randomized to eat the same exact meal with carbs first followed by protein and vegetables or vice versa. Again, while the actual diabetes risk reduction and long-term implications remain to be determined, the study showed that both blood sugar and insulin levels were lower after meals that started with protein and veggies before carbs, compared to eating carbs first.
Finally, vinegar shots before meals have a surprising amount of literature investigating their effect on blood sugars. A recent meta-analysis of controlled trials investigating the impact of vinegar consumption on post-meal glucose and insulin levels suggests that consuming one to two tablespoons (15-30 mL) of vinegar with or shortly before a carbohydrate-containing meal lowers the overall glucose response by an average of 60% and lowers the overall insulin response by an average of 130% compared to the same meal without vinegar. Notably, subgroup analysis suggested that both healthy and insulin resistant people observed a significant benefit, although the effect was more pronounced in people with insulin resistance.
If we combine all this knowledge, it isn’t difficult to come up with a basic insulin-sensitive meal plan (that, honestly, may reduce the palatability of the meal). Evidence suggests that consuming a high-protein diet in which the meat-based sources of protein are boiled, poached, stewed, or steamed rather than fried, baked, or grilled may be able to increase insulin sensitivity. Additionally, consuming protein- and fiber-rich vegetables with vinegar before starchy carbohydrate meals is likely to have a positive effect on insulin sensitivity.
While the type of dietary protein may not have significant effects on insulin sensitivity, simple dietary changes can, such as cooking meats with gentle rather than harsh methods, eating starchy carbohydrates last in a meal (i.e., after protein and fibrous vegetables), and taking a shot of vinegar with or before the meal.
Frequently Asked Questions
Q. Do animal and plant proteins differ in other ways?
Plants are lower in protein content, and therefore require greater consumption to obtain the same amount of most amino acids that would be obtained in a serving of animal-based protein. There are also issues with the amino acid profile and digestibility of many plant proteins that may impede their ability to support bodily growth and repair.
It’s also important to note that not all sources of protein are the same, even within the broad categories of plants and animals. But generally speaking, plant proteins have lower digestibility unless heavily processed (e.g., plant-based protein powders), and have a reduced ability to stimulate protein synthesis and promote muscle growth because of their lower leucine and essential amino acid content. They also do not always contain the full spectrum of essential amino acids and may therefore contain limiting amino acids that impede protein synthesis.
Despite the notable differences in protein quality, research suggests that the ingestion of greater quantities of plant-based proteins may compensate for their deficits. However, there are still the issues of caloric intake, feasibility, and cost to consider.
What should I know?
Although the insulin sensitizing effects of high-protein diets in people with type 2 diabetes are well established, less is known about how the protein composition of the diet influences insulin sensitivity. In the study under review, people with type 2 diabetes consumed a weight maintenance diet providing 30% of the calories as protein, 30% as fat, and 40% as carbohydrate for six weeks. One group consumed most their protein from dairy products and meat while another group consumed most of their protein from foods enriched with pea protein.
Using the gold-standard hyperinsulinemic-euglycemic clamp technique, the study found no significant differences between groups for insulin sensitivity. There were also no differences between groups for blood pressure, blood lipids, serum markers of glycemic control, C-reactive protein, and blood and urinary markers of kidney function. This suggests that the amino acid composition of high-protein diets does not have a notable influence on the studied health markers in people with type 2 diabetes.
The use of functional foods enriched with pea protein in this study limits the external validity of the findings since most people obtain plant protein from actual plant foods that also contain fiber and bioactive compounds that can provide health benefits. However, this study directly tested and answered the question of whether the amino acid composition of plant- and animal-based proteins influences their effect on insulin sensitivity.
