For well over a thousand years, cast iron has been used as a reliable cooking surface. Actually, it's been used since the appropriately named “Iron Age,” roughly 2500 years ago.
Fast forward a couple millennia, and we’ve been thrust into the “Spend all day on the Internet Age”. People are starting to question the healthiness of everything, including the venerable cast iron. And not without reason: just because cast iron’s been used for years, by many people who lived long and healthy lives, doesn’t necessarily mean it’s the healthiest option for your frying pan.
Let’s explore this issue in depth. What exactly is cast iron? Are there any plausible mechanisms by which it might harm health? What does the research say?
Paying the iron price
Cast iron is relatively easy to analyze, as far as health effects go. It’s made out of … iron. Not like the more complex pans, which have multiple layers or man-made coatings. So let’s start by talking a little bit about iron.
You can’t just dig up pure iron from the ground. Pure iron is rare and mainly comes from fallen meteorites. And it's actually pretty soft, so not great for making pans without adding in some carbon for hardening. But still, around 97-98% of a cast iron pan is plain ol’ iron, which is why we’re so interested in its health effects. Our discussion also applies to carbon steel cookware (such as woks), which is made up of 99% iron.
Other than being such an important material for making pans and skyscrapers, iron is also an essential dietary mineral. And cooking on a cast iron pan can transfer some of that mineral from the pan to the food to your body. Some will see this as a good thing, especially considering that 1.6 billion people around the world are anemic, with iron deficiency being the main cause.[1] But iron-deficiency anemia in the US is much less common, with 5 million people having iron-deficiency anemia.[2]
So when most people think of iron, they think of getting enough iron. Iron is one of the few nutrients that a doctor will ask you about, and iron even takes up one of the coveted nutrient spots on the US nutrition label! But the same property that makes iron so useful in the body, its ability to give or receive electrons, makes it potentially harmful when you ingest too much, due to increased production of free radicals.[3][4]
Too much iron has been linked to a wide variety of conditions, such as Alzheimer’s, heart disease, and colorectal cancer to name just a few.[5][6][7] There’s a couple groups of people who don’t have to worry quite as much about iron overload though: menstruating women and vegetarians/vegans. But for others, especially those who regularly eat red meat, it doesn’t take much to push yourself into excess iron territory.
Chances of iron overload in different people
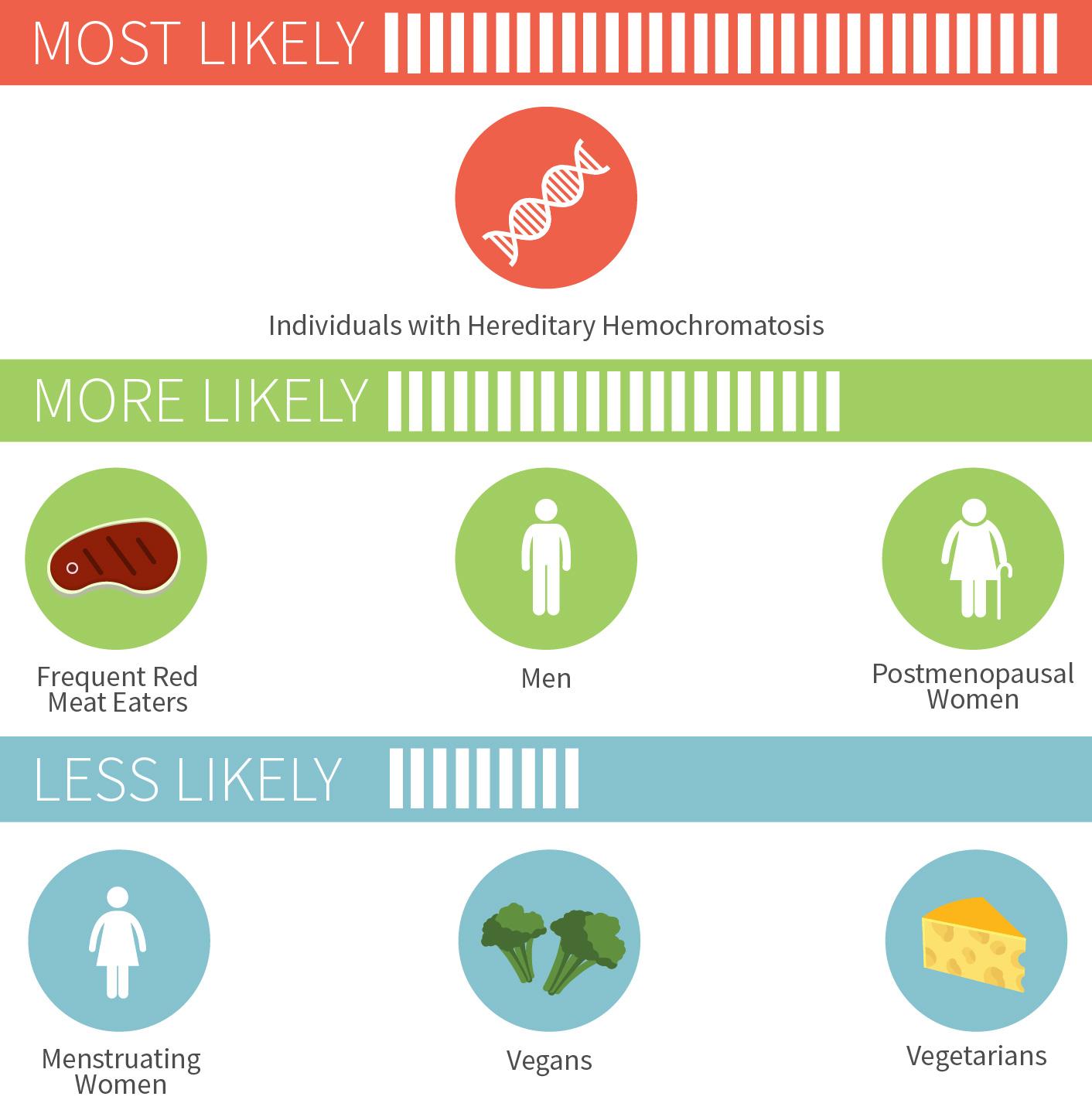
For the nearly one million Americans who have hereditary hemochromatosis, a condition that typically emerges in adulthood where you absorb too much dietary iron, the risk is much more serious. As is the risk of iron poisoning in children, which started being recognized in the 1980s and led to removal of iron from some children’s multivitamins.[8][9]
Certain multivitamins and foods (especially breakfast cereals) have iron added to them, which can contribute to iron buildup over time in those that don’t get rid of iron once a month, namely all males and all non-menstruating females. One of the most popular multivitamins on pharmacy shelves has 100% of the iron RDA per pill, and that one extremely popular breakfast cereal (the one with the bee who’s always up in your business for some reason) contains almost 50% of the iron RDA. A bowl or two of cereal a day, plus red meat a few times a week, a few iron-fortified energy bars, a multivitamin… the iron can add up really quickly.
There are three general strategies for limiting iron. The first is to simply eat less of it, like by switching to an iron-free multivitamin (if you choose to take one at all). Second, you can take advantage of the various iron-absorption inhibitors, such as coffee and certain plant phytochemicals.[10] The third method seems like the most radical option: donate blood every once in awhile, and ditch some of the iron trapped in your red blood cells.[11]
Note that while regular blood donation helps both you and others, and is quite effective at normalizing iron levels according to a randomized trial,[12] it's not always well tolerated.[13] Other than reducing iron in the diet, certain supplements may be able to reduce iron levels, and curcumin has shown efficacy specifically for iron overload.[14]
How much iron are we talking?
We’re talking a pretty decent amount of iron, depending on the condition of your cast iron pan, and what specifically you’re cooking.
To put it into context: men need 8 mg of iron a day, and a serving of tomato sauce cooked in a cast iron skillet can provide 5 mg of iron![15]
Iron content of foods cooked in a cast iron pan
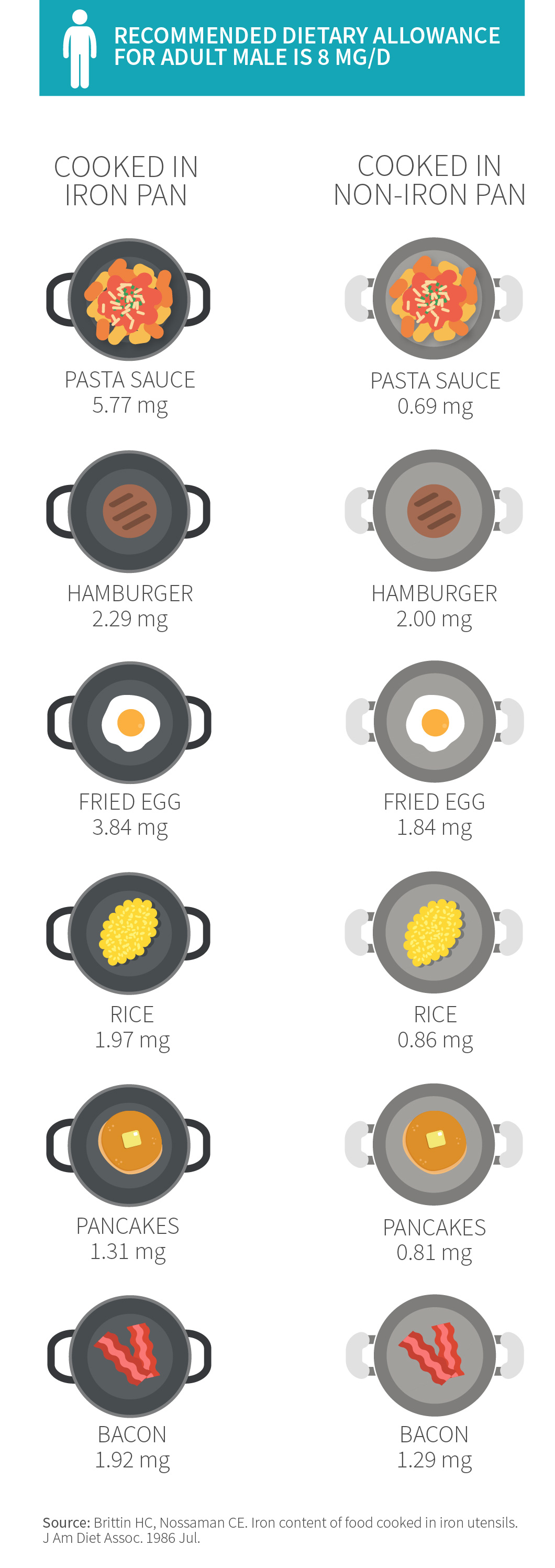
This effect is so reliable that iron cookware has occasionally been used to combat anemia. A variety of studies have shown that iron pots and pans can boost your iron status, substantially increasing the iron content of certain foods (like eggs and applesauce) but not others (like hamburgers).[16][17] The pan’s iron is in the non-heme form, which isn’t absorbed as well as heme iron from meat. But vitamin C can greatly increase absorption, as can acidity, so recipes containing things like lemon or tomato sauce can boost absorption.
If you don’t want your cast iron pan to leach so much iron, make sure it’s well seasoned. Since acidic foods help transfer iron from the pan into your food, you want to put a barrier between the acid and the iron. And that barrier is seasoning, which we’ll talk about in the next section. A pan that is newer and more likely to stick food will also leach more iron than an ancient and heavily seasoned pan. Three other factors that cause more leaching are: using liquid, increased cooking time, and mixing the food more often.
Some of you may be wondering why cast iron (and carbon steel) are uniquely susceptible to this leaching process. What about stainless steel? Steel is made out of iron, after all.
Well, the key to the anti-stain property of stainless pans is chromium, which makes up about 10% or more of the pan. A thin layer of chromium oxide makes stainless steel pans moisture and rust resistant, unlike cast iron pans which can rust very easily. While moisture finds it hard to get INTO the stainless steel pan, a side-benefit is that iron finds it hard to get OUT of the pan. So iron leaching isn’t a big concern here.
That doesn’t mean stainless steel is 100% safe for everyone. While iron overload is a risk that applies to many millions of people, a far smaller number of people are allergic to nickel and chromium, and both of these metals can theoretically leach from stainless steel pans.[18][19] For people with severe nickel or other metal allergies, an enameled pan may be a safe bet.
Cast iron seasoning … aka oxidizing oil on purpose
Aside from iron, there are two other possible dangers of using cast iron pans. One is that you could drop a heavy pan onto your foot or hurt your wrist maneuvering it around. This is only partially a joke, as some cast iron pans can get EXTREMELY heavy compared to all other types of pans. The other possible danger is only theoretical at this point: the risk from eating tiny bits of flaked-off seasoning from the pan’s surface.
So what exactly is seasoning? Well, cast iron pans can easily collect moisture and develop rust. To prevent that, and also get a nice non-stick finish, you have to season the pan with oil rich in polyunsaturated fats. Ideally, you’d continue to season it over the course of years, with more usage adding to the seasoning layers. Like wine and cheese, cast iron is one of the few things that get better with time.
The chemistry is pretty simple. First, get a largely unsaturated fat, like flaxseed oil. For the same reason you don’t want to overload your body with a ton of polyunsaturated fats, that they’re easily oxidized, these fats can be useful on cast iron. When exposed to high heat on top of iron, which acts as a catalyst, the unsaturated fatty acids oxidize, then polymerize into a coating that fills in pores. Further heating carbonizes/hardens the coating.
A well-seasoned pan will appear deep black, and will be almost non-stick. Pans that are pre-seasoned in the factory are not actually well-seasoned; they’re just seasoned enough to prevent rust. You have to keep adding thin layers of fat over time to get that perfect seasoning, since attempting to add one thick layer all at once will result in a greasy pan, with largish pieces chunking off. And it takes a certain heat range to form a good seasoning layer. Really high heats (like above 500° F) will burn off all the seasoning, while low heats (like less than 300° F) won’t encourage enough polymerization of the fatty acids.
The pros of the seasoning process are numerous: you can eventually cook eggs without them sticking, you don’t have to re-season as often, the pan won’t rust, and you can get much cooking cred from your foodie friends.
The cons are harder to quantify. Bits of the seasoning will come off over time (and be replaced by more seasoning). Nobody knows exactly how much comes off over time, nor do they know what the health effects are of eating tiny bits of this type of broken down fat. If you heat the pan up fairly high over long periods, might carcinogenic fumes or free radicals develop from the oxidized oil?[20] Would small amounts of these hypothetical byproducts even be of concern, given the natural antioxidant defenses our bodies employ?
Despite this uncertainty, you shouldn’t be overly alarmed. The flaxseed oil seasoning on your cast iron pan may be oxidized, but it’s not rancid — meaning, it doesn’t impart undesirable odors or flavors (for the most part). This may seem confusing at first, since all the double bonds in a bottle of flaxseed oil mean that it can go rancid easily when not refrigerated. This is because the double bonds are easily attacked by air and light, among other factors.
But when you season your pan using flaxseed oil and heat, the double bonds don’t get randomly attacked. Rather, the double bonds in the flaxseed oil open up and form bonds with neighboring flaxseed fatty acids, with the help of iron and air. It’s a delicate game — gently heating a pot of flaxseed oil would be a recipe for rancidity, but doing it in a thin layer with the help of a really hot iron pan and air… that creates the oh-so-useful seasoning.
Given the lack of certainty about health effects, it’s easy to get alarmed. But if you want to get really up in arms about seasoning, you’d better make sure to also stay away from other heated foods that contain known carcinogens, like the acrylamide in browned potatoes and in breakfast cereals, heterocyclic amines in cooked meat, etc etc.[21][22][23] Remember that toxicity lies in the dose. You don’t have to boil or steam all your foods in order to live a long and healthy life.
I hate science, just tell me if I should ditch my cast iron
If cast iron has been used for so many centuries, and hasn’t shown obvious harm, why even question it at all? There are at least two good reasons.
First, many other types of pans are available, and it’s a buyer’s market. Cast iron is actually not an optimal material for many types of cooking (which we’ll get into in a second), so you definitely don’t need to own a cast iron pan.
Second, and most important, people often cook with their pan on a near-daily basis. Over the years, that adds up to a lot of exposure to whatever the pan gives off.
Cast iron is great for a lot of reasons. It’s cheap, it can and will last a lifetime and get better with age, and you can safely throw it into a super hot oven. All that heavy iron also means that these pans retain heat really well, so they excel in tasks like searing a thick and juicy steak. Some other, thinner pans wouldn’t do as well, since a cold steak would drop down the pan’s temperature upon contact. Another benefit is that the fairly-nonstick nature of cast iron pans will still allow it to develop a “fond” (which is French for “base” or “foundation”) on the bottom, if you happen to enjoy making delicious fond-based sauces.
On the con side, cast iron is extremely heavy and not that easy to take care of (at least until it’s older and well-seasoned). Carbon steel pans are similar in function, but around 25% lighter. Plus they’re a bit smoother, which makes them slightly more non-stick than cast iron, unless you sand down your cast iron pan like some cooking fanatics do. But cast iron isn’t actually a very good heat conductor, so it’s prone to developing hot-spots and cold-spots. Not good if you want even cooking. And since carbon steel is thinner, it’s even more prone to uneven heating.
Another con has to do with our old enemy (or friend if you’re anemic), leached iron. If you make a tomato sauce using a cast iron pan, and it tastes weird, there’s a chance the leached iron is the culprit. Again, seasoned pans are your friend, both for non-stick purposes and for avoiding off-tastes in cooked food.
These cons can be mitigated by using more than one type of pan. Different pans are good at different things, so having more than one pan around might be a good idea if you’re into cooking. Even the much-hated Teflon pan is often used by discerning chefs to make eggs. It doesn’t leach anything under normal heat conditions, and even ingesting tiny amounts of Teflon shouldn’t really harm you, since it’s inert. Heating a Teflon pan under high heat for long periods though … those toxic fumes aren’t great for your health and especially your bird’s health.[24][25]
Even with all the options available, there is no perfect pan, given the wide variety of factors people look at. These include non-stickiness, searing ability, ease of use, even heating, and so on and so on. Cast iron does well with some of these and poorly with others.
We might never know the exact health effects of cast iron pans. Eating bits of oxidized oil every day might seem unwise, but a perhaps more likely detriment is getting too much iron, especially when using a newer or less-seasoned pan.
The decision to choose cast iron or a different cooking material depends on a variety of personal preferences, including risk aversion, what you enjoy cooking with, and what you already own. Trying out more than one type of pan may be wise, or even using more than one type of pan on a regular basis, depending on what’s best suited for the job at hand.