Table of Contents
Protein Intake Calculator
Pound (lb)
Kilogram (kg)
at least —
grams/day.
How much protein do you need per day?
As with most things in nutrition, the answer can be rather complex. Your ideal intake of calories and protein depends on your health, body composition, main goal, and the type, intensity, duration, and frequency of your physical activity. And even taking all this into account, you’ll end up with a starting number, which you’ll need to adjust through self-experimentation.
Nonetheless, as a basic starting point, generally healthy people (i.e., those without a condition that may necessitate limiting protein intake) should aim for a protein intake of at least 1.2 grams per kilogram of body weight per day (g/kg/d), regardless of their body composition and physical activity levels. Women who are pregnant or lactating should aim for a protein intake of at least 1.7 g/kg/d.
Daily protein requirements should be based on body weight, not caloric intake, and are expressed in grams, either per kilogram of body weight (g/kg) or per pound of body weight (g/lb). Ranges in the table below reflect known individual variances.
| Optimal daily protein intake for adults based on body composition goal (g/kg) | ||
|---|---|---|
| Healthy body weight | Overweight/obese | |
| Maintenance | ≥1.2 | ≥1.2 |
| Muscle gain | 1.6–2.2 | 1.2–1.6* |
| Fat loss | 1.6–2.4 | 1.2–2.4* |
* The upper end of the range should be consumed to maximize increases in muscle mass, based on limited evidence
Optimal daily protein intake for healthy, sedentary adults
For adults, the US Recommended Dietary Allowance (RDA) for protein is 0.8 g/kg/d.[2] However, a more appropriate statistical analysis of the data used to establish the RDA suggests this number should be higher: 1.0 g/kg/d.[3]
Note that, contrary to popular belief, the RDA doesn’t represent an ideal intake. Instead, it represents the minimum intake needed to prevent malnutrition. Unfortunately, the RDA for protein was determined from nitrogen balance studies, which require that people eat experimental diets for weeks before measurements are taken. This provides ample time for the body to adapt to low protein intakes by down-regulating processes that are not necessary for survival but are necessary for optimal health, such as protein turnover and immune function.[4]
An alternative method for determining protein requirements, called the Indicator Amino Acid Oxidation (IAAO) technique, overcomes many of the shortcomings of nitrogen balance studies.[5] Notably, it allows for the assessment of protein requirements within 24 hours, thereby not leaving the body enough time to adapt. Studies using the IAAO method have suggested that about 1.2 g/kg/d is a more appropriate RDA for healthy young men,[6] older men,[7] and older women.[8][9]
Further evidence that the current RDA for protein is not sufficient comes from a randomized controlled trial that confined healthy, sedentary adults to a metabolic ward for 8 weeks.[10] The participants were assigned to one of three hypercaloric diets, all of which provided 40% more calories than needed to maintain body weight, but which differed in protein content: 0.7, 1.8, or 3.0 g/kg/d. Despite the fact that a hypercaloric diet has been shown to increase lean body mass,[11] lean body mass actually slightly decreased in the low-protein diet group, while lean body mass increased in the two higher-protein diet groups.
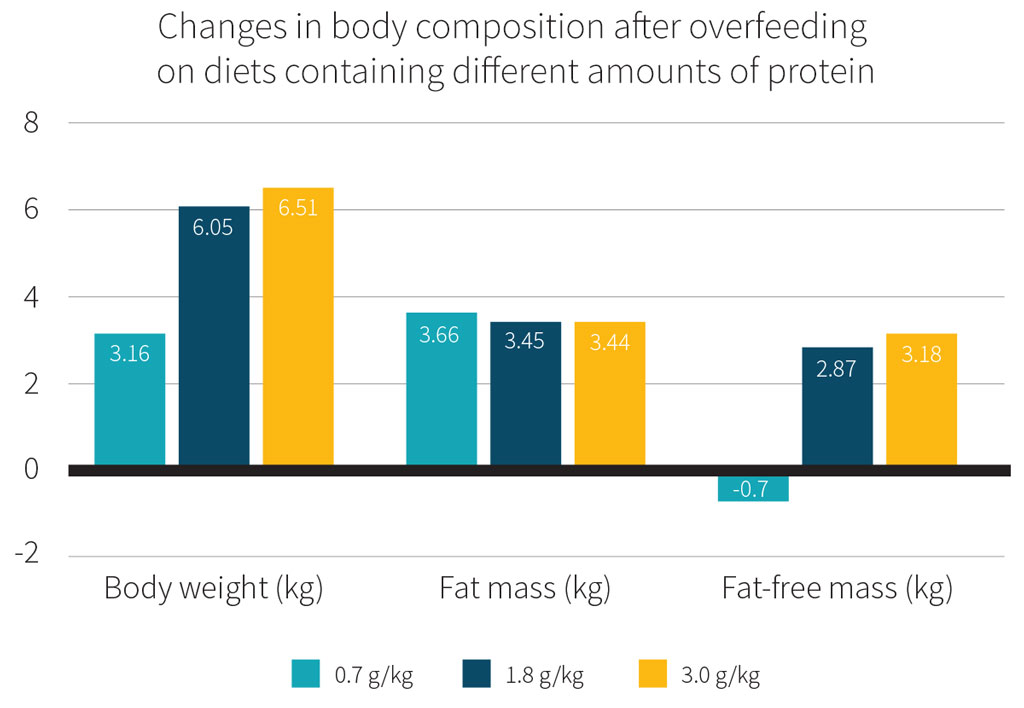
Therefore, we recommended that healthy, sedentary adults without any particular body composition goals aim for a daily protein intake of at least 1.2 g/kg/d to support overall health.
Of note, only a few studies have looked at very high-protein diets (≥2.8 g/kg/d). While these studies demonstrated that very high-protein diets are safe in healthy individuals,[12][13][14][15] further studies with longer intervention durations are needed.
Optimal daily protein intake for athletes
The American College of Sports Medicine, the Academy of Nutrition and Dietetics, and the Dietitians of Canada recommend 1.2–2.0 g/kg/d to optimize recovery from training and to promote the growth and maintenance of lean mass when caloric intake is sufficient.[16] This recommendation is similar to that of the International Society of Sports Nutrition (ISSN), which is 1.4–2.0 g/kg.[17]
However, accumulating evidence suggests that athletes should aim for the higher end of the above ranges. In fact, in certain contexts, it might even be best to go beyond these ranges.
It is important for athletes, especially those who compete in sports involving weight divisions, to optimize their ratio of strength, power, or endurance to body weight in order to gain a performance advantage.[18] In the case of strength and power athletes, this generally entails maximizing the amount of muscle mass on their frame, as a larger muscle has greater force-generating capacity.[19][20] In the case of endurance athletes, this typically means reducing fat mass without sacrificing muscle mass in the process.[21]
A 2018 meta-analysis reported that a protein intake averaging 1.6 g/kg/d maximized resistance-exercise-induced gains in lean body mass;[22] importantly, the upper end of the 95% confidence interval was 2.2 g/kg/d, which suggests that some individuals may benefit from consuming more than 1.6 g/kg/d. In addition, athletes may benefit from protein intakes as high as 2.4–2.7 g/kg/d during periods of caloric restriction.[23][24]
Other evidence from IAAO studies also suggests that protein intakes on the higher end of, or beyond, current recommendations may be advantageous. On training days, active women required 1.4–1.7 g/kg/d.[25][26] In comparison, male endurance athletes required 2.1–2.7 g/kg/d the day after a training session;[27] male amateur bodybuilders required 1.7–2.2 g/kg/d two days after a training session;[28] and resistance-trained men required 1.6–2.4 g/kg/d on a training day.[29]
Based on the available evidence, whether an athlete is pursuing weight gain to maximize the accretion of muscle mass, or they’re after high-quality weight loss (i.e., the loss of fat mass while preserving or even increasing muscle mass), a protein intake of at least 1.6 g/kg/d appears to be best,[18] with the potential for further benefit up to approximately 2.7 g/kg/d,[23] depending on the athlete’s body composition goal and the phase of training.
Optimal daily protein intake for muscle gain
Resistance exercise is, of course, required for muscle gain; you can’t just feed your muscles what they need to grow, you also need to give them a reason to grow.[30]
According to the most recent evidence, individuals interested in muscle gain should aim for a protein intake of 1.6–2.2 g/kg/d.[22][31][32].
Don't expect too much, though. The additional muscle gains from consuming more protein are relatively small:
- A 2018 meta-analysis of randomized controlled trials that were 13 weeks long, on average, reported that higher protein intakes enhanced muscle gain by about 0.3 kg.[22]
- A 2020 meta-analysis of randomized controlled trials that were 20 weeks long, on average, reported that higher protein intakes enhanced muscle gain by about 0.48 kg.[31]
- 2022 meta-analysis of randomized controlled trials, most of which were 8–12 weeks long, reported that higher protein intakes enhanced muscle gain by about 0.6 kg.[32]
While it’s true that higher protein intakes enhance resistance exercise-induced muscle gain, more is not necessarily better. Some evidence suggests a threshold value of around 1.6 g/kg/d, on average; that is, increasing protein intake beyond this point does not promote further muscle gain in most people (see below table).[22][32]
Optimal daily protein intake for muscle gain
 Adapted from Morton, RW et al., 2018, Br Jour Sports Med
Adapted from Morton, RW et al., 2018, Br Jour Sports Med
A common question is whether the protein intake recommendation of 1.6–2.2 g/kg/d also applies to people with obesity who are interested in muscle gain, as calculated protein intake will be relatively high and make up a larger proportion of daily energy intake in this population. The short answer is that there currently isn’t any direct evidence to indicate that the optimal daily protein intake for muscle gain differs in people with obesity. There’s also good mechanistic reason to think that the optimal daily protein intake for muscle gain does not differ between lean individuals and those with obesity.[36] Nonetheless, for practical purposes such as being able to consume a more flexible diet, it may be best to aim for the lower end of the recommended range (i.e., 1.6 g/kg/d).
Lastly, it should not go unmentioned that while consuming more protein than 1.6–2.2 g/kg/d may not enhance muscle gain in people consuming an energy-sufficient or hypercaloric diet, it may help to minimize fat gain. Studies investigating changes in body composition in response to overfeeding suggest that protein intakes of 2.4 to 4.4 g/kg/d may help minimize body fat gain.[37]
Optimal daily protein intake for fat loss
In order to maximize fat loss, a hypocaloric diet (a diet in which fewer calories are consumed than expended) must be consumed. However, the downside to hypocaloric diets is that they can lead to a significant loss of lean body mass (15% or more of total weight loss),[38] especially in the absence of resistance exercise.
Furthermore, the more severe the caloric deficit, the greater the loss of lean body mass tends to be,[39] and the leaner the individual, the more susceptible they are to the loss of lean body mass.[24] For these reasons, it is recommended that athletes wishing to lose fat and preserve lean body mass use a conservative approach and lose weight gradually at a rate of about 0.5% to 1.0% per week.[39]
During caloric restriction, rates of muscle protein synthesis (MPS) are lower in both the fasted and fed states, resulting in a net negative protein balance.[40] Consuming a high-protein diet helps preserve lean mass during caloric restriction by stimulating MPS and promoting a positive or neutral net protein balance.
In one study, resistance-trained men consumed a hypocaloric diet which provided 40% less energy than their bodies needed, with either 2.3 or 1.0 g/kg/d of protein. Compared to the low-protein group, the high-protein group had less lean body mass loss (−0.3 vs. −1.6 kg) and similar fat loss (−1.4 kg).[41] In other studies that had resistance-trained participants or athletes consume hypocaloric diets, a protein intake of 1.6–2.0 g/kg/d was found to preserve or even increase lean body mass.[42][43]
Overall, the current evidence indicates that athletes and lean individuals should aim for a protein intake of 1.6–2.4 g/kg/d to maximize fat loss and minimize lean body mass loss, skewing toward the higher end of this range. Intakes above 2.4 g/kg/d don’t appear to have an additional lean-body-mass-sparing effect, however.[24]
Individuals with overweight or obesity often have the goal of losing fat, given that weight loss is associated with an improvement in health outcomes, and high-protein diets are extremely effective for accomplishing this goal for a variety of reasons. For one, high-protein diets help to prevent declines in resting energy expenditure (REE) during weight loss, namely through an increased thermic effect of food and attenuated loss of lean body mass.[44] They also promote greater satiety (“fullness”) and less hunger when compared to lower-protein diets.[45][46]
Two studies demonstrated that the consumption of a high-protein diet (1.6 g/kg/d) during caloric restriction resulted in a superior ratio of fat mass loss to lean body mass loss compared to a low-protein diet (0.8 g/kg/d).[47][48]
Furthermore, meta-analyses have concluded that in people with overweight or obesity, higher-protein diets are superior for weight loss, fat loss, lean body mass maintenance, and improving cardiometabolic risk factors (i.e., triglycerides, blood pressure).[49][50][51] In adults with overweight or obesity, a diet containing 1.07–1.6 g/kg/d (1.25 g/kg/d, on average) of protein led to greater fat loss and lean mass preservation than an energy-matched low-protein diet containing 0.55–0.88 g/kg/day.[52]
 Adapted from Wycherley, TP et al., 2012, Am J Clin Nutr
Adapted from Wycherley, TP et al., 2012, Am J Clin Nutr
Thus, for adults with overweight/obesity who are eating a hypocaloric diet and wishing to lose fat, a protein intake between 1.2–1.6 g/kg/d (0.55–0.73 g/lb) seems to be optimal, at least based on the overall body of evidence, and a continued protein intake within this range will also support long-term weight-loss maintenance.[44]
There is a lack of research examining protein intakes above this range in people with overweight or obesity, which limits confidence in the idea that higher protein intakes would be even more beneficial in this population. However, there is one study that found that a protein intake of 2.4 g/kg/d markedly improved body composition during a severe caloric deficit, compared to a protein intake of 1.2 g/kg/d.[53] It therefore seems reasonable to suggest that a protein intake of 2.4 g/kg/d may help individuals with overweight or obesity to optimize body composition during caloric restriction.
Thus, individuals who are interested in losing fat while also maximizing muscle gain in the context of a hypocaloric diet may want to aim for a daily protein intake between 1.6–2.4 g/kg/day. It should be noted that there may be practical constraints to achieving this intake in individuals with a higher body weight: someone weighing 100 kg would need to consume up to 240 grams of protein per day!
Optimal daily protein intake for older adults
Sarcopenia is a progressive and generalized skeletal muscle disorder that increases the risk of disability, poor quality of life, and mortality. It is characterized by a decline in muscle strength and muscle mass, as well as impaired physical performance in severe cases. The revised 2019 definition of sarcopenia by the second European Working Group on Sarcopenia in Older People (EWGSOP2) places an emphasis on muscle strength, in contrast to earlier definitions which prioritized muscle mass,[54] because loss of muscle strength seems to be a more reliable predictor of adverse outcomes. Furthermore, strength is lost 2–5 times faster than muscle mass.[55]
A 2022 review estimated that the global prevalence of sarcopenia in adults younger than 60 and adults 60 or older ranged from 6% to 36% and 10% to 27%, respectively.[56] Sarcopenia prevalence seems to be the greatest in Oceania and lowest in North America when using the European Working Group on Sarcopenia in Older People (EWGSOP) definition.[56]
The most common cause of age-related frailty and sarcopenia is muscle loss. Older adults tend to lose muscle mass at a rate of about 0.5%–1% per year, muscle strength at a rate of about 1%–3% per year, and power at a rate of about 3%–4% per year after the age of 50.[57] The loss of muscle strength accelerates around age 75, and may decline by 3%–4% each year.[55][58] This is why building muscle throughout early and midlife is crucial, and why minimizing age-related muscle loss/maintaining strength is a huge priority.
 Adapted from Cruz-Jentoft, AJ et al., 2019, Age and Ageing
Adapted from Cruz-Jentoft, AJ et al., 2019, Age and Ageing
What is causing this muscle loss? One factor may be the typical decline in physical activity and exercise levels as people age — most individuals don’t exercise as much as they did when they were younger. There’s also evidence to suggest that not only do older individuals eat less protein than they should, but that their bodies are less responsive to protein — a phenomenon known as “anabolic resistance”. Put simply, if a young person and an older person both consume 20 grams of protein, the younger person has a greater muscle protein synthetic (MPS) response to that protein.[59][60]
For this reason, it’s recommended that older adults prioritize protein intake, and the amount will differ depending on one’s goals and unique circumstances:[61]
- Sedentary but healthy older adults should aim to consume at least 1.2 g/kg/d (0.54 g/lb/d)
- Older adults who are sick, injured, or undergoing periods of immobilization or inactivity due to surgery or bedrest should aim to consume ≥1.6 g/kg/d (≥0.73 g/lb/d)
- Older adults wishing to lose weight or highly active adults wishing to build muscle should aim to consume 1.6–2.4 g/kg/d (0.73–1.1 g/lb/d)
Finally, while it appears that increasing protein intake to between 1.2 and 1.6 g/kg/d can maintain and increase muscle mass in most older adults, increasing protein intake alone may not be enough — adequate protein intake should also be combined with resistance training.[32]
Optimal daily protein intake for pregnant women
Pregnancy is a dynamic, anabolic state involving changes in metabolism[62] and increases in whole-body protein turnover, protein synthesis, and tissue accretion,[63] which influence dietary protein requirements. Adequate dietary protein intake during pregnancy is essential for optimizing pregnancy outcomes. For instance, protein supplementation during pregnancy has been found to reduce the risk of stillbirth and small-for-gestational age and increase birth weight,[64] particularly in undernourished women.
The protein RDA for pregnant women is 1.1 g/kg/d.[2] This value was estimated by adding three values:
- The RDA for a healthy adult (0.8 g/kg/d)
- The amount of additional body protein a pregnant woman accumulates
- The amount of protein used by the developing fetus
However, as seen previously with non-pregnant healthy adults, the RDA may not be sufficient, let alone optimal. There’s some evidence from studies using the IAAO technique that the requirement for some amino acids is higher than the recommended amounts during certain parts of pregnancy.[65][66]
According to one study, the RDA for pregnant women should be about 1.66 g/kg/d during early gestation (weeks 11–20) and 1.77 g/kg/d during late gestation (weeks 32–38).[67]
Keep in mind that the above is simply what’s been reported in studies; it’s not possible to know whether the data are directly applicable to any given individual’s health and pregnancy. Furthermore, these values are derived from women in the general population carrying one child, so it’s possible that women who are highly physically active or are supporting the growth of more than one child need even higher amounts.
As always, an obstetrician/gynecologist (OB/GYN) should be consulted before making any dietary changes.
Optimal daily protein intake for lactating women
As with pregnancy, there is little research investigating how lactation and breastfeeding affect protein requirements.[68] Women produce a wide range of breast milk volumes, regardless of their energy status (e.g., milk production is maintained even in women with a BMI under 18.5).[69] The infant’s demands appear to be the primary regulator of milk production.[70][71]
Based simply on adult protein requirements plus the protein output in breast milk, the RDA for lactating women was set at 1.3 g/kg/d.[2]
However, one study reported that half of the lactating women consuming 1.5 g/kg/d were in negative nitrogen balance,[72] while another study suggested that 1.0–1.5 g/kg/d leads to a rapid downregulation of protein turnover suggestive of an adaptive response to insufficient intake.[73]
There’s also preliminary evidence from a study using the IAAO technique that suggests exclusively breastfeeding women (3–6 months postpartum) need 1.7–1.9 g/kg/d.[74]
In combination, these data indicate that lactating women should aim to consume at least 1.7 g/kg/d, but further evidence is needed to define the optimal daily protein intake in this population, and to investigate how carrying more than one child may alter this value.
Optimal daily protein intake for infants and children
| Preterm infants | Infants (0-6 months) | Infants (7-12 months | Toddlers (1-3 years) | Children (4-13 years) | |
|---|---|---|---|---|---|
| Grams of protein per kilogram of body weight per day | 3.0-4.0 | ≥1.5 | ≥1.2 | ≥1.05 | ≥1.55 |
Optimal daily protein intake for infants
Healthy infants
The adequate protein intake for healthy infants aged 0–6 months, based on their average weight and milk intake, is 1.52 g/kg/d.[2]
The average protein intake of healthy infants aged 7–12 months is estimated at 1.6 g/kg/d,[75] assuming that half their protein comes from breast milk and half from complementary foods. Yet the RDA is set at 1.2 g/kg/d for this age group, based mostly on studies conducted in toddlers and children.[76]
Preterm infants
Preterm infants need to be fed enough protein to promote growth rates similar to those observed in healthy fetuses growing in utero. The following daily intakes have been recommended based on gestational age:[77]
-
3.5–4.0 g/kg/d (≤ 30 weeks)
-
2.5–3.5 g/kg/d (31–37 weeks)
-
1.5–2.0 g/kg/d (>37 weeks)
Moreover, a systematic review by the Cochrane Collaboration reported greater weight gain and higher nitrogen accretion in preterm infants whose protein intake was 3.0–4.0 g/kg/d, compared to lower daily intakes.[78]
Since breast milk doesn’t contain enough protein to meet these requirements, complementary supplementation is standard practice.[79][80]
Formulas and complementary feeding
Breast milk is considered the optimal source of nutrition for infants 0–12 months old, and is recommended as the exclusive source of nutrition for non-preterm infants aged 0–6 months.[81] However, not all infants can breastfeed. Infant formulas provide an alternative, but there are considerable differences in composition from breast milk.[82] One such difference is the protein content, which tends to be higher in formula.
Infant feeding has the potential to influence long-term health outcomes. Notably, breastfeeding is associated with a reduced risk of obesity later in life,[83] with a greater duration of breastfeeding associated with a greater reduction in risk.[84]
Rapid weight gain in infancy and the second year of life is associated with an increased risk of obesity later in life,[85][86] and formula feeding is associated with greater weight gain than breastfeeding.[86][87][88] There are a variety of potential mechanisms to explain this association.[89] One of them is that breastfeeding helps the infant learn to better regulate their energy intake.[90]
Another potential reason is the higher protein content in formula. Higher-protein formulas have been shown to produce greater weight gain compared to lower-protein formulas, despite similar energy density.[91]
For instance, in a study that had healthy full-term infants consume either higher-protein formulas (2.9 and 4.4 grams of protein per 100 kcal), lower-protein formulas (1.7 and 2.2 grams of protein per 100 kcal), or breast milk, it was found that weight-for-age, weight-for-length, and BMI at 12 months were greater in the higher-protein group than in the lower-protein group, while length-for-age did not differ between groups.[92] Additionally, there were no differences between the lower-protein and breastfed groups.
Follow-up data from this cohort showed that BMI was significantly higher in the participants in the higher-protein group at 6 years of age and the risk of obesity was 287% higher, compared to the lower-protein group.[93] Meanwhile, there were no differences between the lower-protein and breastfed groups.
In support of these findings, a 2022 meta-analysis reported that higher protein intakes early in life (≤ 18 months) were associated with a higher BMI later in life.[94]
While these data suggest that a higher protein intake early in life may increase long-term obesity risk, it’s possible that the source of protein is driving this increase in risk. In the aforementioned meta-analysis, a higher intake of total animal protein was associated with a higher BMI, while there was no association found for total plant protein.[94]
Moreover, it may be the case that a higher intake of non-breastmilk dairy protein affects growth differently than other animal-based protein sources. In a study that had formula-fed infants consume protein-equated diets (3.0 g/kg/d) that were either meat-based or dairy-based. After 7 months, the meat-supplemented babies averaged longer for their age than when they started, and the dairy-supplemented infants averaged heavier for their age but shorter for their age than when they started.[95] However, it bears mentioning that this study was funded by the National Cattlemen’s Beef Association and the National Pork Board.
In a separate study that had breastfed infants consume either a higher-protein (2.9 g/kg/d) meat-based diet or a lower-protein (1.4 g/kg/d) cereal-based diet for 5 months,[96] length-for-age increased in the meat group compared to the cereal group, without differences in age-adjusted weight-for-length or BMI between groups. This suggests that, in contrast to a higher-protein dairy-based diet, a higher-protein meat-based diet does not lead to unfavorable growth. However, this study also had some limitations. It was deemed to have a high risk of bias due to a significantly lower length-for-age in the meat group at baseline, evidence of selective reporting, and funding from the National Cattlemen’s Beef Association.[97]
In combination, the available evidence suggests that while breastfeeding lowers the long-term risk for obesity, high non-breastmilk dairy protein intake in infancy increases the long-term risk of obesity or excess fat mass.[98]
During their first six months, healthy infants should consume ≥1.5 g/kg/d of protein. This intake can be achieved exclusively through breastfeeding. Preterm infants require 3.0–4.0 g/kg/d to facilitate catch-up growth. From ages 6 to 12 months, infants should consume ≥1.2 g/kg/d, but further research is needed to determine what the optimal daily protein intake is.
Optimal daily protein intake for toddlers
The same data used to establish the RDA for infants aged 7–12 months (1.2 g/kg/d) was used to determine the RDA for toddlers aged 1–3 years: 1.05 g/kg/d.[2]
There is a dearth of data for this age group; further research is needed to determine the optimal daily protein intake for toddlers. Nevertheless, it bears mentioning that the average daily protein intake of US toddlers is estimated to be over three times the RDA (3.5 to 3.7 g/kg/d), with 90% of them consuming at least 2.65 g/kg/d.[99]
Optimal daily protein intake for children
The protein RDA is slightly higher for children (4–13 years) than for adults: 0.95 versus 0.8 g/kg/d. This difference makes sense considering that children are still growing and need more protein to facilitate this process. As with adults, however, the RDA may underestimate true requirements.[2]
Use of the IAAO technique in children aged 6–10 years has suggested that around 1.55 g/kg/d would make for a more appropriate RDA.[100] Protein needs are likely higher in children involved in sports and other athletic activities, relative to their sedentary peers, due to enhanced muscle protein synthesis and breakdown.[101] However, the amount of additional protein that may be needed is unknown.
Notably, observational studies typically show that the average protein intake among children in developed countries is two to three times the RDA.[102] For instance, the average daily protein intake of US children (ages 4–8) is estimated to be 2.5–2.76 g/kg/d.[103]
More research is needed to determine an optimal daily protein intake for children, and whether protein recommendations should differ for children involved in sports.[102] Nonetheless, the best evidence available suggests that at least 1.55 g/kg/d is a solid target to shoot for.
Optimal daily protein intake for vegans
It’s commonly thought that vegans, or people whose protein intake is mostly derived from plant-based sources, require higher protein intakes to achieve their body composition goals because plant-based protein sources are of lower quality than animal-based protein sources.
Protein quality
Protein quality is determined by the protein’s digestibility (i.e., the amount or proportion of dietary-protein-derived amino acids that are made available in a form suitable for body protein synthesis)[104] and amino acid profile. The Digestible Indispensable Amino Acid Score (DIAAS) is currently the preferred method for determining protein quality.[105]
The main strengths of the DIAAS, compared to the previous method used to determine protein quality (the Protein Digestibility Corrected Amino Acid Score, or PDCAAS), is that it considers the digestibility of individual essential amino acids (EAA) and is based on ileal digestibility (the disappearance of a nutrient between the mouth and the end of the small intestine), as opposed to fecal digestibility (the disappearance of a nutrient between the mouth and the end of the digestive tract).[105] The DIAAS is based on the content of the most limiting EAA in a protein source; that is, the EAA present in the lowest amount in the food.
The DIAAS may be below or above 100%, with 100% indicating that the dietary protein provides all of the EAA in adequate amounts; more specifically, it means that if the food is consumed in an amount equivalent to the estimated average requirement (EAR, the amount of a nutrient estimated to meet the requirement 50% of healthy people) for protein (0.66 g/kg/d), amino acid needs will be met.
Therefore, a DIAAS of 90% means that if one were to consume the EAR for protein exclusively from that protein source, only 90% of the daily requirement would be met for the most limiting EAA in that protein source.[106] On the other hand, a DIAAS of 110% means that ingestion of the protein source would supply 110% of the daily requirement for the most limiting EAA, and more than 110% for all the other EAA.[106]
 Adapted from Herreman L, et al., 2020, Food Sci Nutr
Adapted from Herreman L, et al., 2020, Food Sci Nutr
The supposed inferiority of plant-based proteins
Muscle protein synthesis (MPS) is primarily driven by the postprandial rise in plasma EAA levels,[107] and leucine is a particularly important EAA in this equation, as it triggers or “turns on” the MPS process.[108][109] Plant-based protein sources are thought to provide a lesser anabolic stimulus than animal-based protein sources because they generally contain lower amounts of EAA overall and often contain inadequate amounts of one or more specific EAA, typically lysine, methionine, and/or leucine. All amino acids are required for MPS,[110] and thus a lack of one or more amino acids may compromise the MPS response.
 The dashed line represents the amino acid requirements for adults; adapted from Pinckaers PJM, et al., 2021, Sports Med
The dashed line represents the amino acid requirements for adults; adapted from Pinckaers PJM, et al., 2021, Sports Med
Another reason that plant-based protein sources are considered inferior is due to their lower digestibility, which is largely a consequence of the presence of “antinutritional factors,” i.e., compounds such as phytates, saponins, and tannins which are present in whole plant foods and that interfere with digestion and absorption of the available protein. However, plant-based protein powders — being mostly free of these compounds — are about as digestible as animal-based protein sources.[111]
In addition to digestibility issues, some evidence suggests that a lower proportion of dietary-protein-derived amino acids from soy protein is available for MPS compared to milk proteins, due to the amino acids in soy protein being more readily taken up by the gut and liver and converted to urea.[112][113][114]
Acute MPS studies
Mechanistically, it seems to be an open-and-shut case that plant-based protein sources provide a lesser anabolic stimulus than animal-based protein sources. However, this is mostly based on DIAAS, which was designed by the Food and Agriculture Organization to help the global population avoid malnutrition, not as a measure to determine the anabolic potential of dietary protein sources.
While the DIAAS accounts for the relative digestibility of EAA, it does not consider more downstream physiological targets of interest to a physically active person who wants to improve their body composition, such as the stimulation of MPS.[115] Therefore, to gain a more accurate understanding of the anabolic potential of a dietary protein sources, it’s critical to combine assessments of protein quality with direct measures of MPS.[115]
A handful of studies have directly compared the MPS response following the ingestion of a plant-based and an animal-based protein source.
In young, healthy adults, although the ingestion of 18–22 grams of soy protein has been shown to stimulate MPS less than the same amount of protein from whey or milk (but not casein),[116][117] when the same of amount of protein was combined with 45 grams of carbohydrate — which arguably is more indicative of the type of mixed macronutrient meal that people typically consume — MPS rates were similar between groups.[118]
However, when the dose of protein is bumped up to 30 g, plant-based protein — irrespective of whether it’s derived from wheat, potato, corn, pea, or a combination of sources — has effects on MPS rates and anabolic signaling pathways that are comparable to those of whey or milk protein.[119][120][121][122][123] Similar results were also reported in a study that compared chicken breast to a lysine-enriched meat alternative composed of wheat and chickpea protein.[124]
Collectively, these data suggest that a high-protein vegan diet that contains a variety of protein-rich plant foods would stimulate daily MPS rates as much as an omnivorous diet rich in animal-based protein sources. However, a limitation of these studies is that they used plant-based protein isolates or concentrates, as opposed to whole plant foods, which improves protein digestibility and the absorption of the amino acids that drive the MPS response.[111]
Older adults
It’s prudent to separate the data on the MPS response following the consumption of plant-based and animal-based proteins between younger and older adults (≥ 65 years of age), because older adults are less sensitive to the anabolic effects of protein ingestion,[60][125][126] and thus the source of protein may be more influential in this population.
One study of older adults found that the ingestion of 20 or 40 grams of soy protein did not increase MPS in the resting state, whereas the ingestion of 20 or 40 grams of whey protein did.[112] In the same study, the ingestion of 20 grams of soy protein did not increase MPS following resistance exercise, but the ingestion of either 40 grams of soy protein or 20 or 40 grams of whey protein did.
In another study of older adults that compared the ingestion of wheat protein to whey and casein protein,[127] 35 grams of casein protein increased MPS in the resting state, but 35 grams of wheat protein or whey protein did not. However, 60 grams of wheat protein was found to increase MPS.
In combination, these data suggest that, compared to an animal-based protein source, higher doses of protein from plant-based sources may be required to stimulate a robust MPS response in older adults.
Longer-term studies
With respect to longer-term studies, which measured MPS over a duration longer than a few hours, or better yet, measured changes in muscle mass over a few months — as acute changes in MPS are not necessarily indicative of changes in muscle mass over time[128] — there are two main studies to consider in young adults.
Both studies included a resistance exercise intervention and randomized the participants to consume a high-protein diet (≥1.6 g/kg/d), either mostly from animal-based sources or exclusively from plant-based sources, and both found comparable increases in muscle mass between groups after a few months.[129][130]
An important detail in both of these studies is where the participants got most of their protein from in the vegan group. In one study, over half of the participants’ protein intake was from soy, which, unlike other plant-based protein sources, is considered an “excellent quality” protein (meaning it has a DIAAS ≥100[131]) that meets the EAA needs of adults.
In the other study, over half of the participants’ protein intake was from mycoprotein derived from the fungus Fusarium venenatum. Mycoprotein is another unique plant-based protein source in that it is relatively rich in EAA and, in particular, in leucine.[132] It’s also been shown to stimulate MPS rates to a greater degree than milk protein.[133]
In sum, the available evidence in young adults indicates that as long as total daily protein intake is sufficiently high, and a variety of plant-based protein sources are consumed, with an emphasis on higher-quality sources, vegans do not need more protein than omnivores to build muscle.
Unfortunately, there is an absence of studies like those mentioned above in older adults. At present, the only longer-term study available compared daily MPS rates over 3 days between a group consuming a high-protein, mycoprotein-enriched (57% of protein intake) vegan diet and a protein-matched omnivorous diet.[134] No difference in daily MPS rates were found between groups. Further research is needed to determine whether high-protein vegan and omnivorous diets have comparable effects on muscle mass in older adults.
Strategies to improve the anabolic properties of plant-based protein sources
The available evidence suggests that, in the context of a high-protein diet (≥1.6 g/kg/d), the source of protein does not materially affect changes in body composition; that is, vegans don’t need to consume more protein than their meat-eating counterparts to build muscle. However, the evidence base is relatively limited, especially when it comes to older adults. As such, it’s worth expanding on strategies to maximize the anabolic potential of plant-based protein sources.[111]
One way to compensate for a limiting EAA is to simply consume a greater amount of protein, as evidenced by the aforementioned study in older adults that found an increase in MPS with 60 grams of wheat protein but not 35 g.[127] However, while this is a feasible strategy in the context of plant-based protein powders, it may be impractical for most whole plant foods.
An alternative, and generally more practical, strategy is to combine plant-based protein sources with different amino acid profiles in a meal in order to obtain adequate amounts of all EAA. For instance, a plant-based protein source low in lysine but high in methionine (e.g., corn, hemp, brown rice) can be combined with a plant-based protein source low in methionine but high in lysine (e.g., pea, soy).
Another way to overcome a limiting EAA and increase the anabolic potential of plant-based protein sources is to supplement with one or more EAA that the protein source has inadequate amounts of. Leucine is a particularly good candidate, as a sufficient amount of leucine is essential to initiate the MPS response to feeding.[135][136] Furthermore, supplementing a suboptimal dose of protein with leucine has been shown to increase MPS as much as an optimal dose of protein.[137] Further research is needed to confirm the efficacy of this strategy, but one study in rodents found that fortifying wheat protein with free leucine to match the amount of leucine in whey protein resulted in similar increases in MPS between groups.[138]
 Adapted from Pinckaers PJM, et al., 2021, Sports Med
Adapted from Pinckaers PJM, et al., 2021, Sports Med
How much protein per meal?
Muscle protein synthesis (MPS) is the process of building new skeletal muscle tissue. When MPS chronically exceeds muscle protein breakdown (MPB), resulting in positive net muscle protein balance, muscle growth occurs over the long term.[128] Each snack or meal represents an opportunity to promote muscle growth, as dietary protein ingestion stimulates MPS.
Protein-feeding studies using various doses of whey protein suggest that 0.24 grams of protein per kg of body weight per meal (g/kg/meal) will maximize the MPS response of the average young adult,[59] whereas 0.40 g/kg/meal will maximize the MPS response of most young adults. For older adults, who are less sensitive to the anabolic effects of protein ingestion, these two values jump to 0.40 and 0.60 g/kg/meal.[59]
This anabolic resistance documented in older adults has also been observed in young adults with obesity.[36][139] Therefore, individuals with obesity whose primary goal is muscle gain should probably aim for a protein dose of at least 0.40 g/kg/meal.
There are at least a couple of good arguments in favor of targeting the higher end of the recommended ranges, and it might even be advantageous to exceed them:
First, the recommended ranges are based on the ingestion of whey protein. Whey protein is unique in that it’s highly bioavailable; quickly digested; and rich in essential amino acid (EAA), especially leucine, which is known to trigger or “turn on” MPS. These attributes suggest that less protein from whey protein may be needed to produce a maximal MPS response compared to other protein sources.
There are two main factors to consider in this hypothesis: (i) whether the protein source (i.e., plant-based vs. animal-based) influences the MPS response to protein ingestion; and (ii) whether the food matrix (i.e., whole foods vs. protein powder) influences the MPS response to protein ingestion.
Evidence suggests that 30 grams of protein from plant-based protein isolates or concentrates stimulates MPS as much as as the same amount of protein from milk protein or whey in young adults.[119][120][121][122][123] However, when lower doses of protein (18–22 g) are consumed, milk protein or whey stimulates a greater MPS response than soy.[116][117]
Therefore, in the context of plant-based protein powders, the higher end of the range should be targeted in order to obtain at least 30 grams of protein. It’s even more crucial to target the higher end of the range for whole plant foods (e.g., beans) because their digestibility tends to be lower than that of animal-based sources,[111] unlike plant-based protein powders which are about as digestible as animal-based protein powders.[111] In fact, for this reason, it may even be beneficial to exceed the higher end of the recommended range when it comes to whole plant foods, depending on the specific foods consumed, but further research is needed to confirm this.
What about animal-based whole foods vs. animal-based protein powders?
The consumption of 20 grams of protein from whey protein has been shown to produce a near maximal MPS response at rest and after exercise in young and older adults.[112][140] In comparison, 30 grams of protein from lean beef was found to maximize the postprandial MPS in young and older adults,[141] and a separate study in young men reported similar postprandial MPS rates after meals containing 30 grams of protein from either milk or beef.[142] In addition, 18 grams of protein from eggs was found to stimulate a robust MPS response.[143]
Studies directly comparing the postprandial MPS response to the ingestion of whey vs. animal-based whole foods are needed, but it may be the case that the postprandial MPS response does not significantly differ between whey protein and animal-based whole foods,[115] particularly when at least 30 grams of protein is consumed.
Second, the recommended ranges are based on the amount of protein needed to maximally stimulate MPS over a 3–4 hour postprandial period. However, the body doesn’t use dietary protein only to make skeletal muscle; all proteins in the body are in a constant state of turnover. Also, the results are not necessarily generalizable to studies that measure MPS over a longer postprandial period.
Following protein ingestion, approximately 50% of available amino acids are taken up by the gut and liver for the purposes of energy production and local protein synthesis.[144] The gut can also store a large amount of amino acids,[145] ready to be used when the body needs them. In addition, available amino acids can be used for the production of purines and pyrimidines (the building blocks of DNA and RNA) and neurotransmitters.[144]
Furthermore, evidence suggests that ingesting protein beyond the amount known to stimulate a maximal MPS response over 3–4 hours results in greater whole-body net protein balance, primarily via greater inhibition of whole-body protein breakdown.
For example, a study using meals with lean beef found that meals containing 40 and 70 grams of protein increased MPS to a similar extent, but whole-body net protein balance was more positive with 70 grams of protein due to a markedly greater suppression of whole-body protein breakdown and a slightly greater increase in whole-body protein synthesis.[146] In another study by the same group, incrementally higher protein intakes (from about 6 to 92 grams of protein) led to greater whole-body net protein balance.[147]
More recently, a 2023 randomized controlled trial identified a very strong correlation between whole-body protein net balance and protein intake: specifically, ingesting more protein in a meal — up to 2.0 g per kg of body mass (a higher amount was not examined) — led to greater whole-body protein net balance.[148]
It may be the case that, over a 3–4 hour postprandial period, a certain amount of protein maximizes the MPS response, and ingesting a greater amount of protein does not further benefit MPS in that timeframe; however, that doesn’t necessarily mean that a greater amount of protein wouldn’t be found to further stimulate MPS, if MPS were measured over a longer postprandial period.
The same 2023 randomized controlled trial referenced above answered this question. It had recreationally-active young men consume either 25 grams or 100 grams of milk protein after performing a bout of resistance exercise and measured MPS for 12 hours. In the first 4 hours, the study found no difference in MPS between protein doses–but from 4 to 12 hours, the MPS rate was 40% higher with 100 grams than it was with 25 grams of protein.[148]
Thus, in the context of consuming a very large amount of protein in a single meal, there is no “excess” protein that is “wasted”, as is commonly proposed. The body breaks down and uses all of the protein consumed, sooner or later, in muscle or elsewhere.
Based on the available evidence, there does not appear to be a practical limit to the total anabolic response — which considers both whole-body and muscle protein synthesis and breakdown — to protein intake with a meal (beyond the physical limits of the GI tract).[145] However, there may still be an advantage to spreading daily protein intake over a few meals for promoting muscle gain,[149] even if it’s only for the practical purpose of making it easier to meet your daily protein goal.
How much protein after exercise?
According to conventional wisdom, there exists a limited period after a workout where the ingestion of certain nutrients results in an enhanced anabolic response, which helps to maximize the muscular adaptations induced by the workout. This period is referred to as the “anabolic window” and is thought to last about an hour after the completion of an exercise bout.[150] In accordance with this theory, it’s common practice to slam a protein shake immediately after a workout.
It’s true that there have been a couple of studies demonstrating superior increases in muscle protein synthesis (MPS) when protein is consumed immediately after exercise compared to delaying ingestion,[150] but these studies utilized an aerobic exercise intervention and had some other limitations (e.g., one was performed in dogs).[151][152] In contrast, it’s been shown that consuming a beverage containing carbohydrate and essential amino acids either one or three hours after resistance exercise results in an equivalent increase in MPS.[153]
Nonetheless, acute measures of MPS do not necessarily correlate with changes in muscle mass over time.[154] Therefore, attention should be redirected to studies that measured changes in lean mass and strength over time, as these are ultimately the outcomes of interest. Evidence from randomized controlled trials that assessed resistance exercise-induced adaptations over several weeks do not support the existence of an anabolic window.[150] Rather than the timing of protein intake, the available evidence indicates that total daily protein intake is the primary determinant of muscular adaptations.[155]
Even if the anabolic window is better described as a garage door,[156] as MPS remains elevated for at least 24 hours after resistance exercise,[157][158], this shouldn't be misunderstood as a reason to delay protein ingestion shortly after a workout. Although there’s nothing special about slamming a protein shake immediately post workout, compared to consuming protein at a different time of day, any extra protein in the postworkout period will contribute to the recommended total daily protein intake of at least 1.6 g/kg.
As far as the optimal dose of protein is concerned, the same recommendations from the “how much protein per meal?” section generally apply to the postexercise period: 0.24–0.6 grams of protein per kg of body weight; and again, it’s recommended that older adults and people with obesity aim for the upper end of the range.
Notably, the type of exercise performed may influence the optimal dose of protein. For instance, while studies that had participants perform lower-body resistance exercise found that single doses of 20 and 40 grams of whey protein promoted similar increases in MPS,[140][159] a study that had participants perform whole-body resistance exercise found that the ingestion of 40 grams of protein stimulated a greater MPS response than 20 grams of protein.[160] It remains unclear whether 30 grams of protein would have stimulated MPS to the same extent as 40 grams of protein following whole-body resistance exercise.
With respect to endurance exercise, a study that had endurance-trained participants perform 90 minutes of continuous endurance exercise in the fasted state, then consume 45 grams of carbohydrate alongside varying amounts of protein, found that while 30 grams of protein stimulated MPS to a greater extent than 15 grams of protein, there was no difference in the rate of MPS between 30 and 45 grams of protein.[161]
Notably, the relative dose of protein required to maximize the MPS response in this study was 0.49 grams per kg of body weight, on average, which is greater than the reported requirement after resistance exercise.[162] Why protein requirements might be greater after endurance exercise is unclear, but it may be primarily related to greater protein breakdown and whole-body oxidative disposal of amino acids during exercise.[163]




